Advances in Animal and Veterinary Sciences
Case Report
Histopathological and Parasitological Study of Blood-Sucking Haemonchus contortus Infection in Sheep
Mani Saminathan1*, Arumugam Gopalakrishnan2, Annamalai Latchumikanthan3, Arockiasamy Arun Prince Milton4, Manivasagam Aravind3, Kuldeep Dhama1, Rajendra Singh1
1Division of Pathology; 2Division of Medicine; 3Division of Parasitology; 4Division of Veterinary Public Health, ICAR-Indian Veterinary Research Institute (IVRI), Izatnagar, Bareilly (UP) – 243122, India.
Abstract | Haemonchus contortus is a most prevalent, highly pathogenic and economically important nematode of small ruminants. A 10 months old non-descript, female sheep was presented with the history of anorexia, weight loss and voiding dark colour diarrheic faeces. On clinical examination revealed severe emaciation, lateral recumbent position, and pale and anaemic mucous membranes. Post mortem examination revealed pale, hide bound carcass and gelatinization of subcutaneous fat. Internal organs were pale, hydrothorax and ascites were noticed. Liver was swollen, hard and multiple different sized calcified nodules were found. Multiple tiny cysts were present in spleen. Abomasal mucosa showed severe congestion, pin point petechial haemorrhages, watery bloody contents with numerous minute hair like H. contortus worms. Small intestinal mucosa was congested and petechial to ecchymotic haemorrhages were noticed. Caecal mucosa was congested; pin-point haemorrhages and few whipworms were found. Histopathological examination revealed haemorrhages, edema, desquamation and eosinophilic infiltration in the abomasal villi. Spleen showed hemosiderosis and encapsulated cyst with homogenous eosinophilic material lined by thick fibrous wall. Liver showed bile duct hyperplasia, degeneration of hepatocyte and mononuclear cell infiltration. Glomerulus showed eosinophilic protenacious edematous fluid and atrophy of the glomeruli. Parasitological examination revealed numerous H. contortus eggs (+++) and adult female was identified by presence of vulval flap and adult male by presence of spicules, ‘Y’ shaped dorsal rays and copulatory bursa. The present study clearly describes the clinical signs, gross lesions, histopathological changes of haemonchosis and parasite morphology which perfectly correlates with the pathogenesis of H. contortus.
Keywords | Haemonchus contortus, Abomasum, Sheep, Histopathology, Parasitological examination
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | December 20, 2014; Revised | January 03, 2015; Accepted | January 04, 2015; Published | January 12, 2015
*Correspondence | Mani Saminathan, Indian Veterinary Research Institute, Izatnagar, Bareilly, India; Email: [email protected]
Citation | Saminathan M, Gopalakrishnan A, Latchumikanthan A, Milton AAP, Aravind M, Dhama K, Singh R (2015). Histopathological and parasitological study of blood-sucking Haemonchus contortus infection in sheep. Adv. Anim. Vet. Sci. 3(2): 99-108.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.2.99.108
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Saminathan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Small ruminants plays major role in the maintenance of rural farmer’s family stability by providing cash income through milk, meat, skin and wool (Lateef et al., 2005). Haemonchus contortus commonly known as twisted stomach worm or barber pole worm, is a most pathogenic blood- sucking gastrointestinal nematode found in the abomasum of small ruminants (Mortensen et al., 2003), notably sheep and goat; but H. placei is usually affect cattle. These nematodes are economically most significant because it causes insidious loss of production, weight loss and even mortality in lambs (Fentahun and Luke, 2012) and important emerging anthelmintic resistance parasite (Mortensen et al., 2003). These parasites are more prevalent in the tropical and warmer temperate countries, particularly where there is good rainfall in the summer season (Sissay et al., 2007; Santín-Durán et al., 2008; Qamar et al., 2009). The acute and debilitating form of disease is most commonly seen in young animals while adult animals are resistant to infection (Onyenwe et al., 2005; Santín-Durán et al., 2008). The most common clinical signs are failure to thrive, weight loss, anemia, hypoproteinemia, sub-mandibular edema (bottle jaw), ascites, diarrhea, lethargy and death (Kelkele et al., 2012; Tehrani et al., 2012).
Adult male worms are 10-20 mm long and uniformly reddish-brown in colour and female worms are 18-30 mm long and are easily identified by the ‘barber pole’ appearance of the white ovaries and uteri twisting for the length of the worm around a red blood-filled intestine (Love and Hutchinson, 2003). The adult worms and fourth stage (L4) larvae are the vigorous blood-sucking nematodes, movement of the worm causes wounds and secretion of anti-coagulants causes continuous haemorrhage from the abomasal wall results in severe anemia and reduced productivity in acute condition (Tehrani et al., 2012). Each worm can suck about 0.05 ml blood per day in sheep (Burke et al., 2007; Ijaz et al., 2009). Therefore in acute condition animals may be found dead without showing any clinical signs. However in chronic condition animals show pale conjunctivae and mucous membranes, diarrhea, lethargy, muscular weakness, and edema particularly on lower mandibular region and lesser extend to ventral abdomen. The diagnosis of haemonchosis is usually based upon clinical signs and faecal examination (Getachew et al., 2007; Tehrani et al., 2012).
A 10 months old non-descript, female emaciated sheep was brought to Clinics of Medicine, IVRI with the history of anorexia, weight loss, and dark colour diarrheic feces voiding for past 2 days and the animal was treated by local veterinarian. On clinical examination, sheep was found to be weak, severe emaciation, lateral recumbent position and pale conjunctival mucous membrane. The animal was died after treatment and sent for Post mortem examination Facility, Division of Pathology, IVRI.
A well detailed post mortem examination of sheep was conducted to know the cause of the death. First, the animal was examined carefully on the external surface of the body and the internal organs were examined, and the organs showing macroscopic lesions were systematically recorded.
The affected organs such as pieces of abomasum and intestine were collected in 10% neutral buffered formalin (NBF) for histo-pathological examination. Other organs like liver, spleen, lung, kidney, heart, lymph node and brain were also collected in 10% NBF. Feces were collected in suitable container in normal saline for identification of eggs. Parasites were collected 10% NBF for specific parasitic species identification.
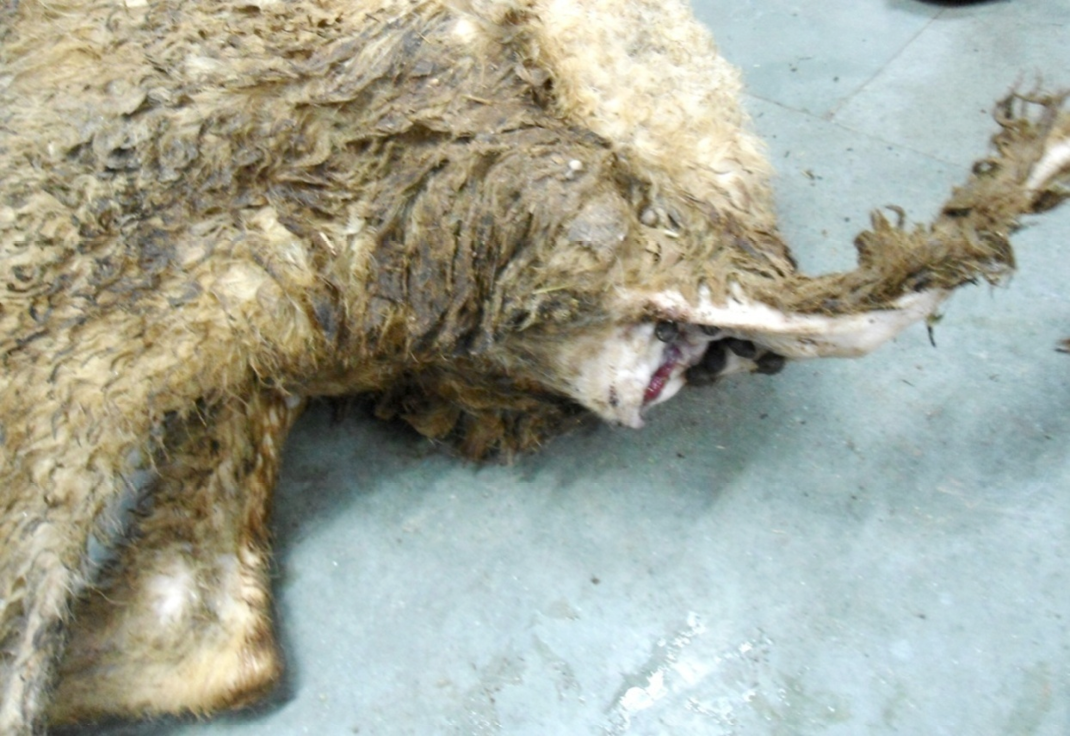
Figure 1: Hind-quarter of the animal is soiled with faeces due to diarrhoea, gaping of the anus and loss of lustre of hairs
It is based on differences in specific gravity of parasite eggs and that of fecal debris. Two gram of the fecal sample was taken in cylinder and 10 ml of flotation solution (saturated salt solution; specific gravity-1.27) was added. An emulsion was made by mixing the flotation solution with the feces. The fecal emulsion was strained through a metal tea strainer into second container and is poured into a 15 ml centrifuge tube. The flotation solution was added until a meniscus is formed in a test tube. A glass cover slip is placed over the meniscus and allowed to remain for 10-15 minutes. The cover slip was removed and placed liquid side down, on a glass slide. The slides were examined under the microscope in low power (10X) for screening of entire area and then high power (40X) objective lens can be used to confirm a diagnosis.
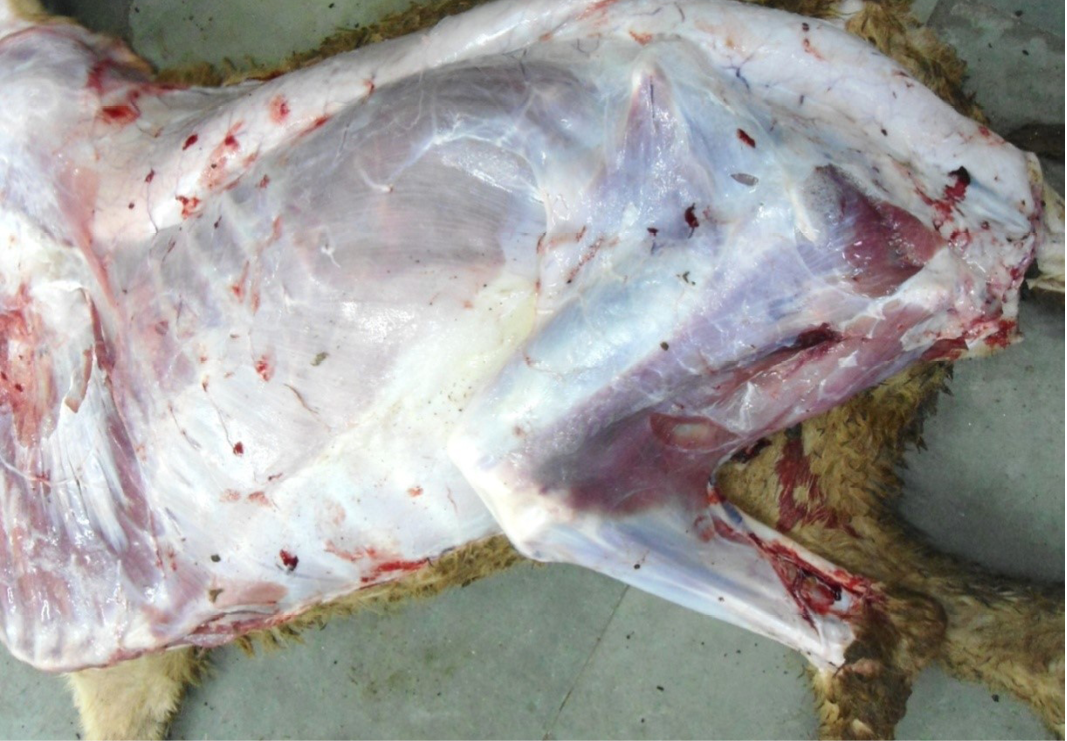
Figure 3: Carcass was pale in colour, hide bound condition, gelatinization of subcutaneous (SC) fat and SC fat was not in appropriate amount
The worms were washed in normal saline solution and processed with lactophenol solution for optimum clearing. Worms were identified under low power microscope based on the standard taxonomical keys provided by Soulsby (1982).
Tissue samples of 1 to 2 mm thickness were dehydrated in graded alcohol and cleared in xylene and embedded in paraffin blocks. The 4-5 µ thick serial sections were taken with rotator microtome on clean grease free slides and subjected for haematoxylin and eosin (H&E) staining (Luna, 1968).
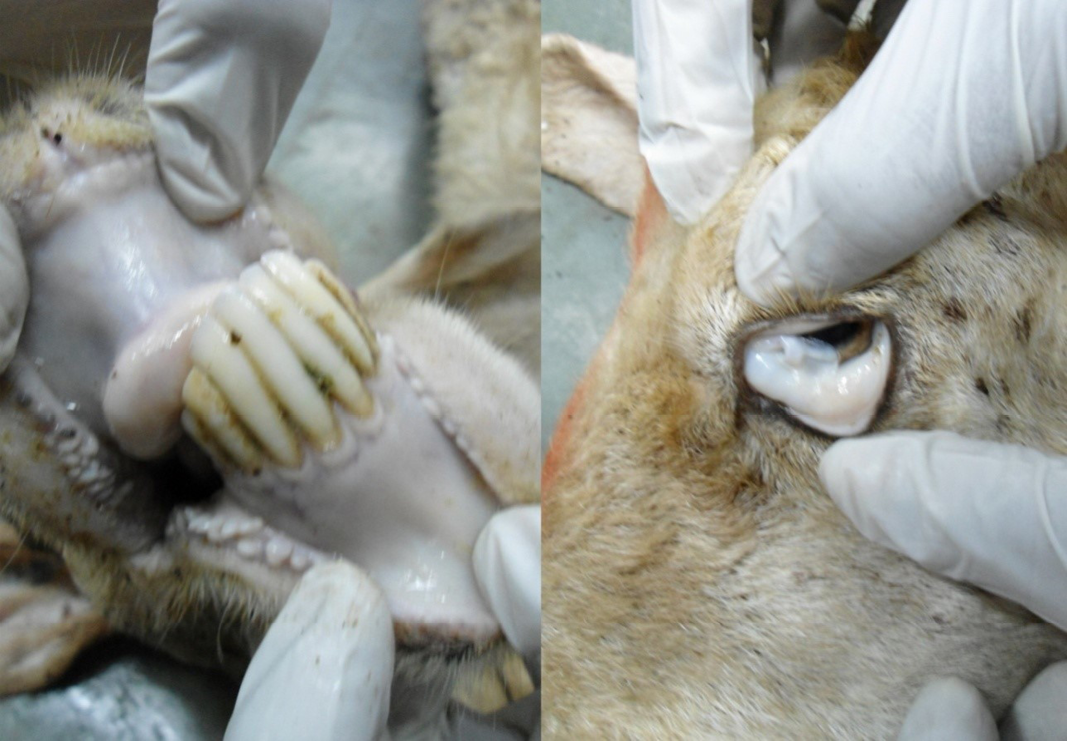
Nutritional status of cadaver was poor (emaciated), dry skin and loss of luster of hairs were noticed. Hind-quarter of the animal is soiled with faeces due to diarrhea and gaping of the anus were noticed (Figure 1). Conjunctival, buccal and vaginal mucous membrane were pale and anemic condition (Figure 2).
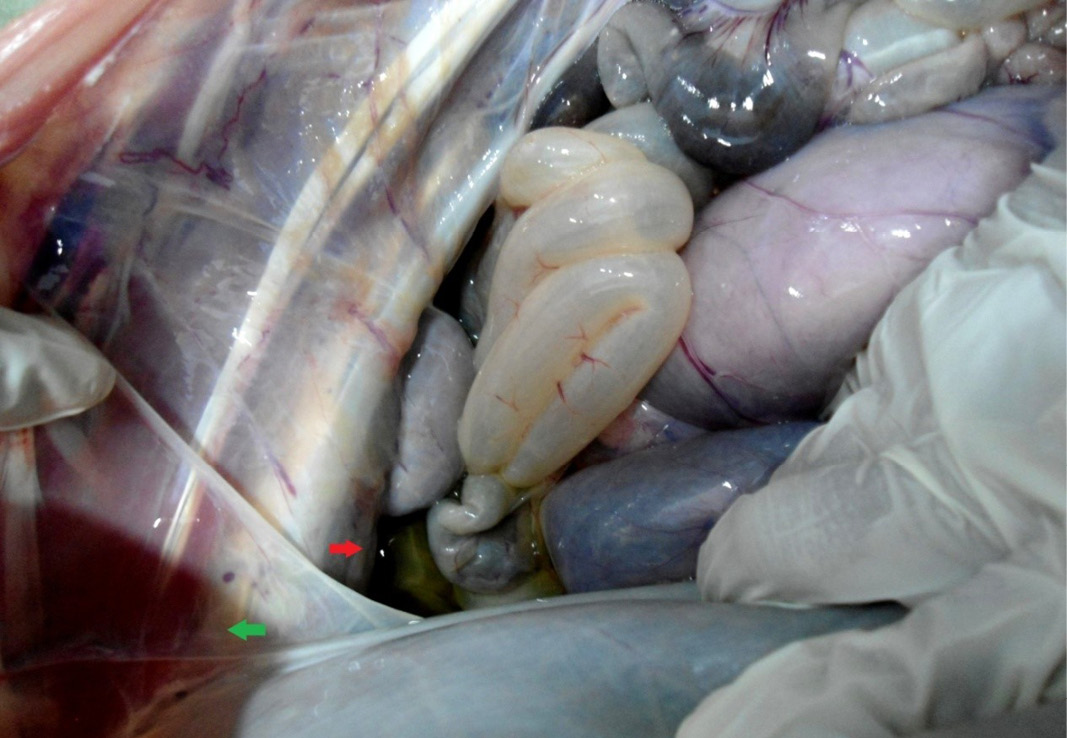
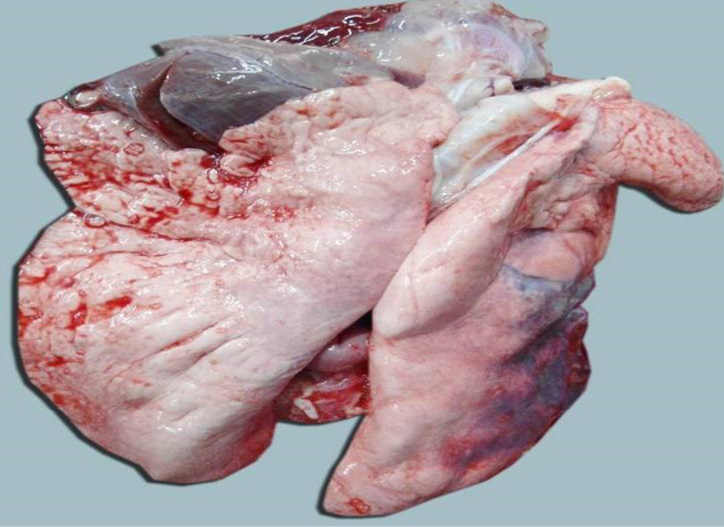
The carcass was pale in colour and hide bound condition (Figure 3). Gelatinization of subcutaneous fat was noticed and subcutaneous fat was not in appropriate amount. The prescapular lymph node was pale, edematous and gelatinization of fat around the lymph node was noticed. The thoracic cavity contains approximately 500 ml of fluid (Figure 4). The abdominal cavity was filled with straw yellow colour fluid (approx. 500-750 ml) (Figure 4). Internal organs were pale in colour. The Lungs were appeared pale and emphysematous and heart was pale and gelatinization of epicardial fat was noticed (Figure 5). Abomasal mucosa was severely congested and showed numerous pin point petechial haemorrages. Numerous minute hair like haemonchous worms (+++) were present in the abomasums (Figure 6). Abomasal contents were watery and partially covered with free blood. Small intestinal mucosa was congested, watery contents, pin-point to ecchymotic hemorrhages (Figure 8) while serosa was pale in colour (Figure 7) and thickened intestinal wall was noticed. Mesenteric lymph node was enlarged, edematous and pale in color (Figure 7). Caecal mucosa was congested; pin-point hemorrhages and few whipworms were attached with mucosa (+) (Figure 9). Multiple tiny cysts filled with straw yellow colour fluid were present in the left lateral border of the spleen (Figure 10). Liver was swollen with rounded borders, hard in consistency and multiple different size calcified masses were noticed on the surface which had produced hard and gritty sound while cutting (Figure 11). Gall bladder was distended with bile and thickening of wall was noticed. Kidneys were pale in color and congested cortico-medullary junction.
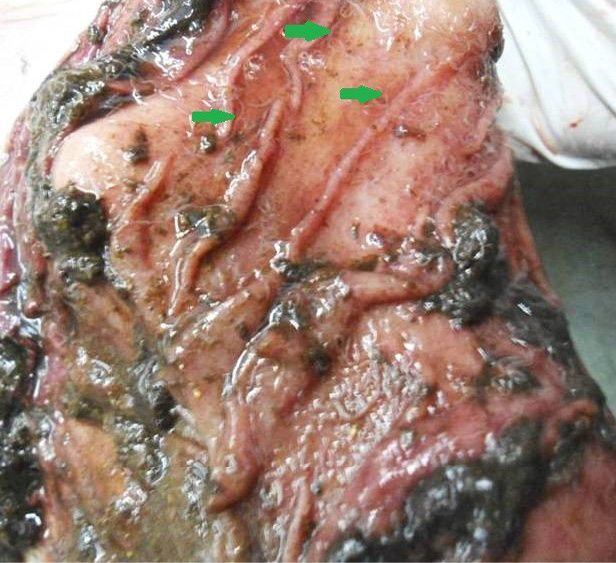
Figure 6: Abomasal mucosa was severely congested; pin point petechial haemorrhages and numerous minute hair like Haemonchus contortus worms (green arrows) were present
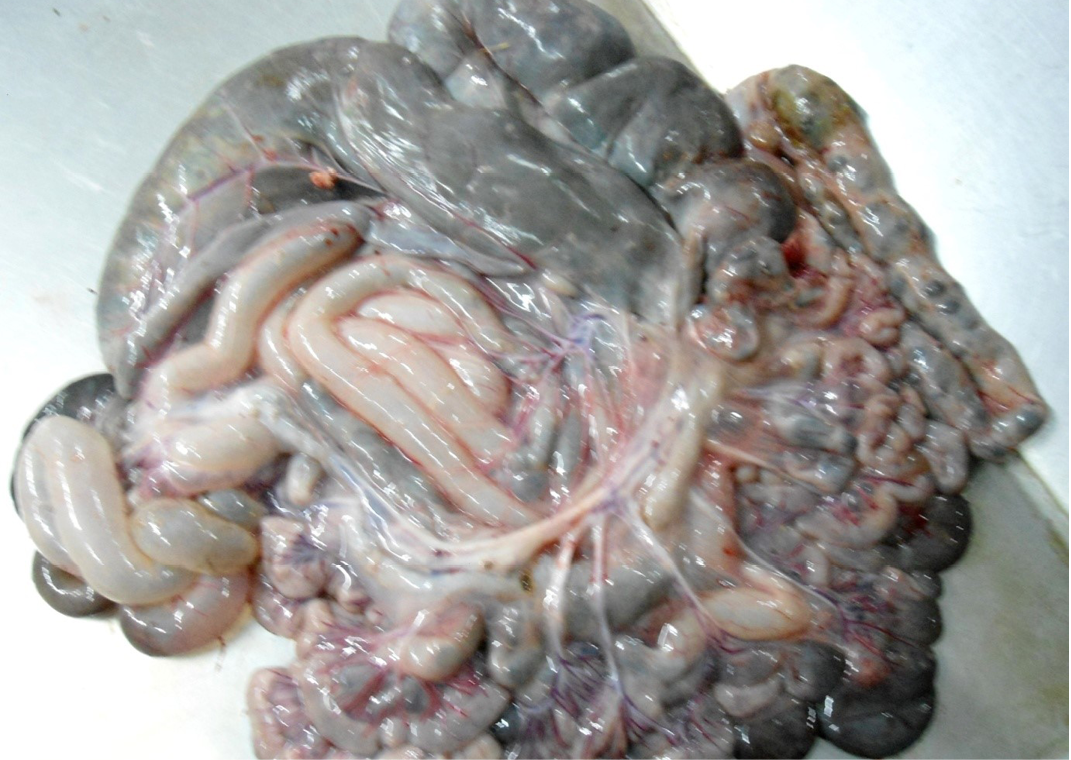
Figure 7: Intestine was pale in colour due to anaemia. Mesenteric lymph node was enlarged, edematous and pale in color
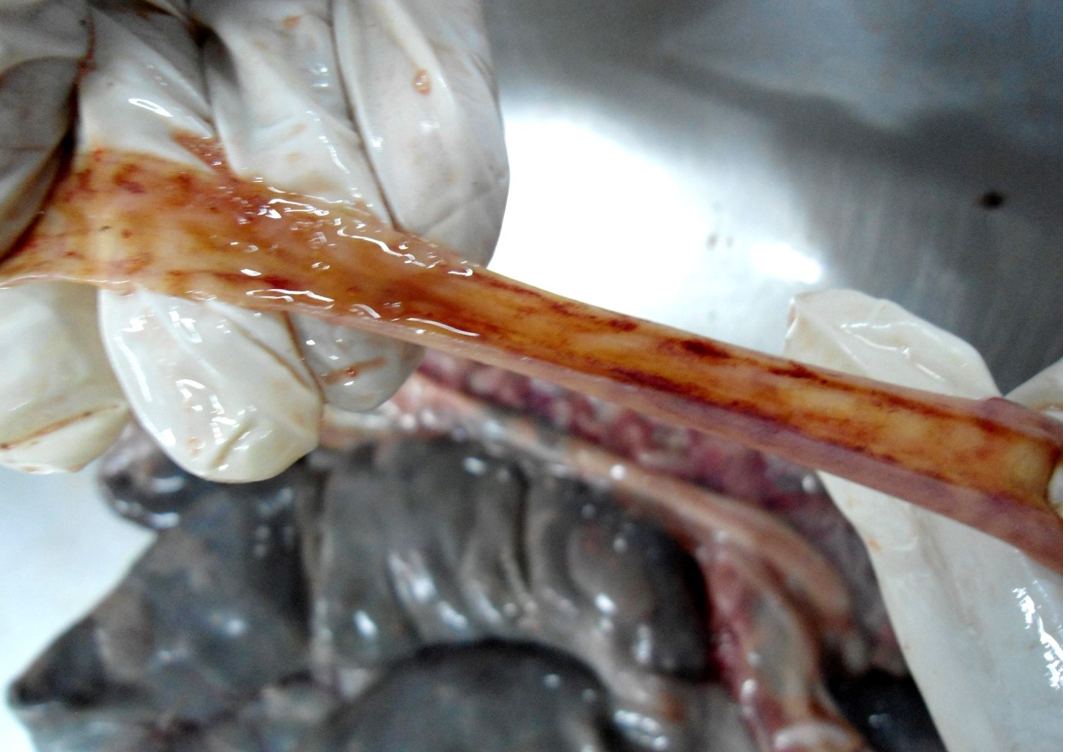
Figure 8: Small intestinal mucosa was congested, petechial to ecchymotic haemorrhages, thickened intestinal wall and watery intestinal contents
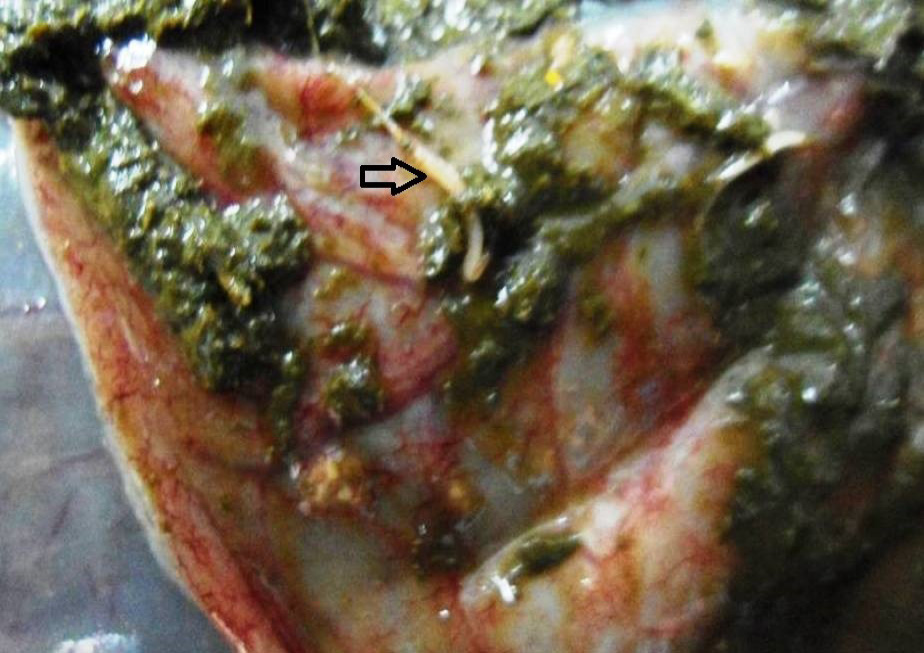
Figure 9: Caecal mucosa was congested; pin-point hemorrhages and few whipworms (arrow) were attached with mucosa
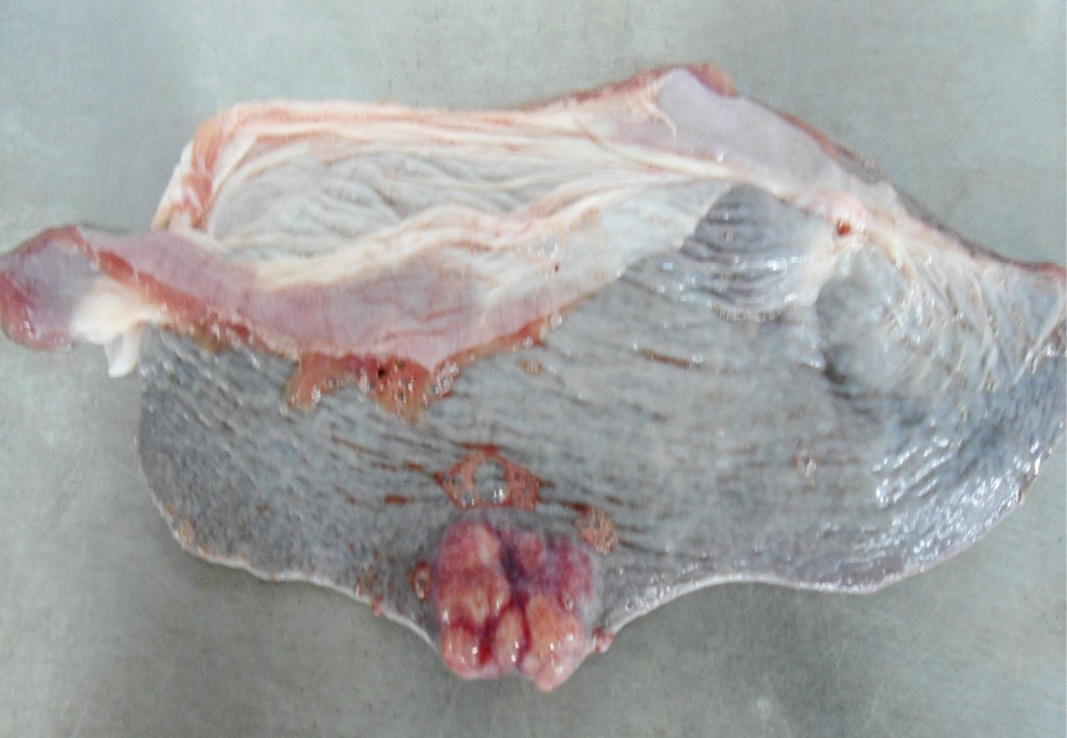
Figure 10: Multiple tiny cysts filled with straw yellow colour fluid were present in the left lateral border of the spleen
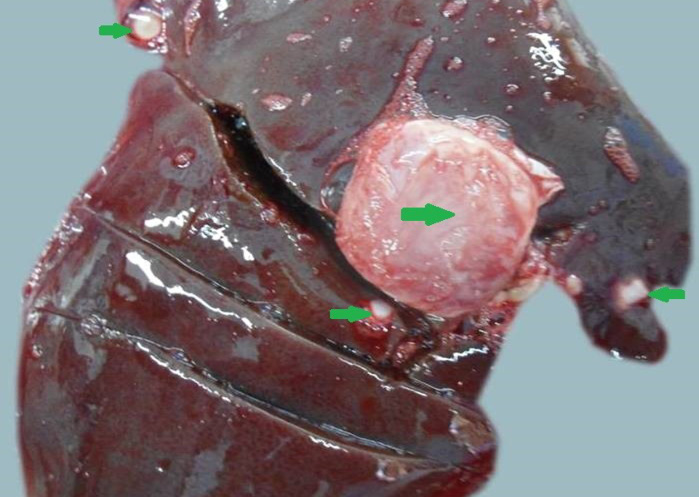
Figure 11: Liver was swollen with rounded borders, hard in consistency and multiple different size calcified masses (green arrows) were noticed
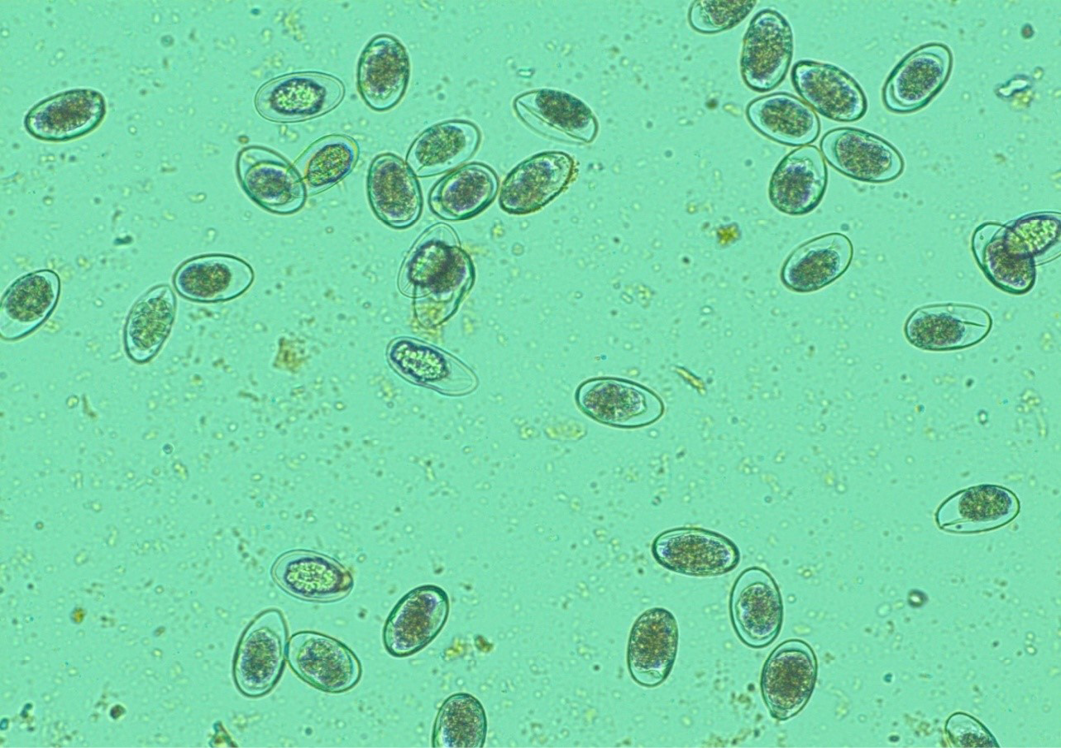
Figure 12: Numerous H. contortus eggs- thin-shelled, oval shape with equal poles, edges mombate and morula not fully filled the cavities of the eggs
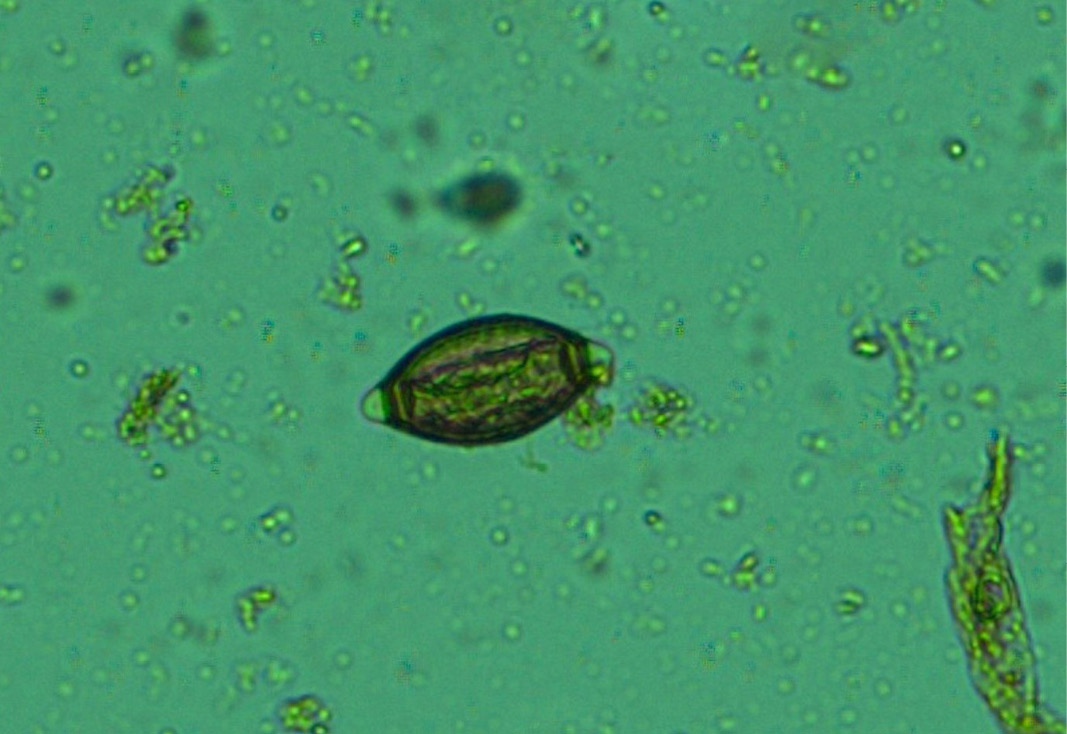
Figure 13: Whip worm (Trichuris ovis) eggs- dark brown colour, thick-walled, barrel shape with unsegmented embryo and transparent polar plugs at both ends
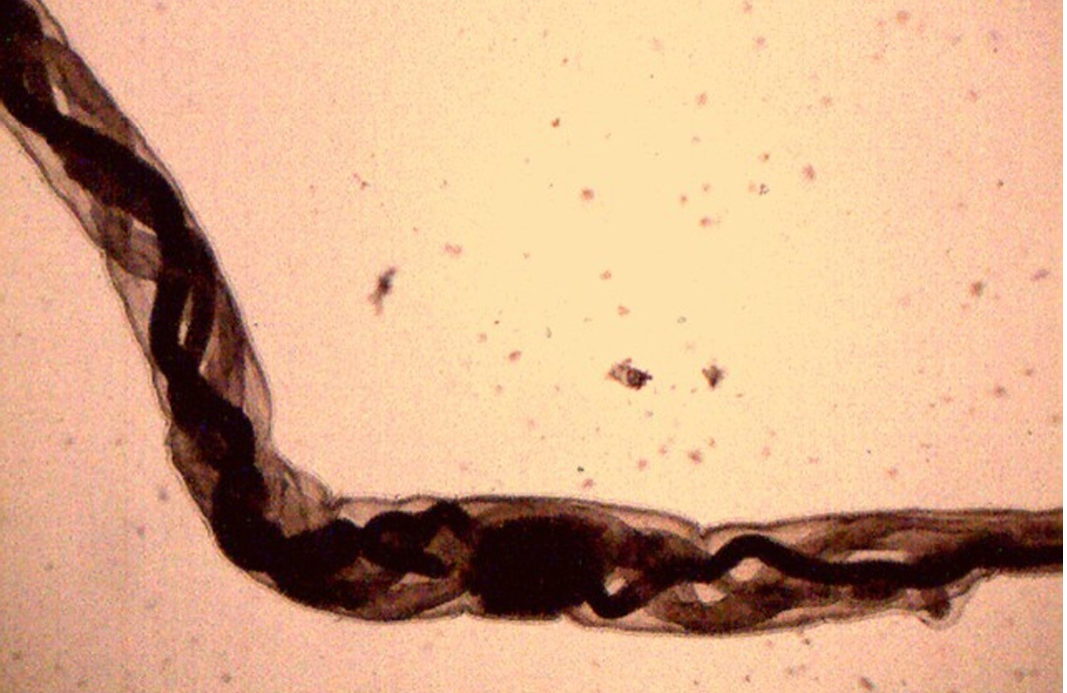
Figure 14: Female H. contortus worms- white uteri and ovaries winding around the red blood-filled intestine give a twisted or barber pole appearance
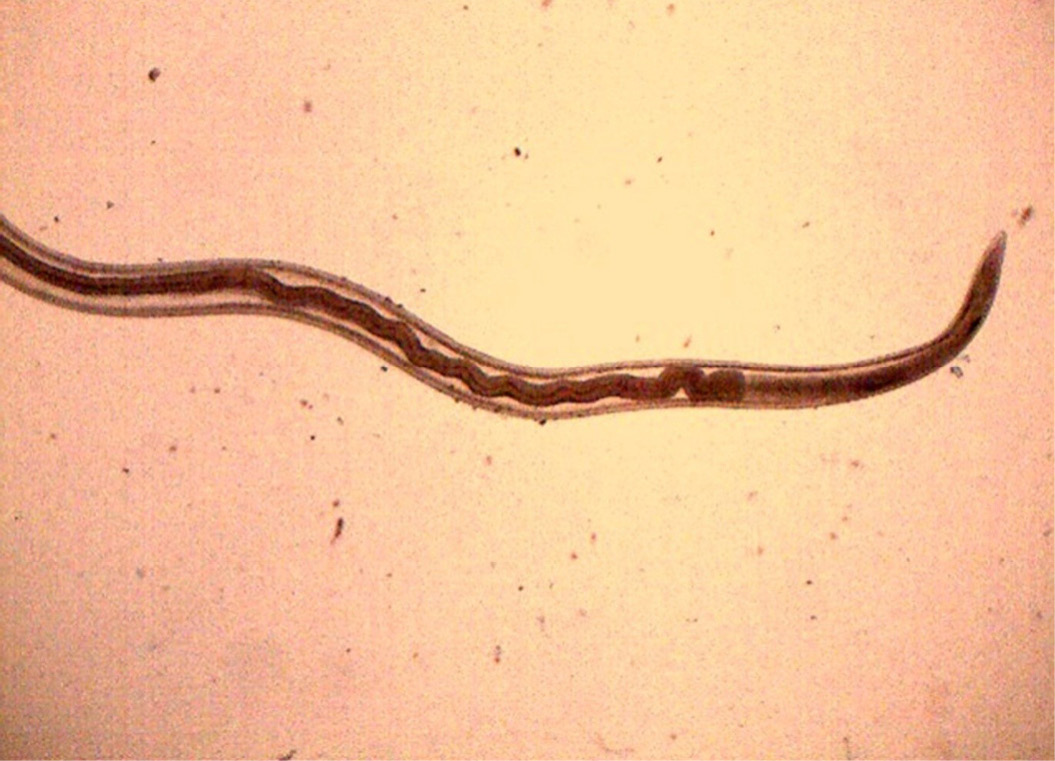
Figure 15: Anterior end of H. contortus was characterized by small funnel shaped buccal capsule and spine like pair of cervical papillae
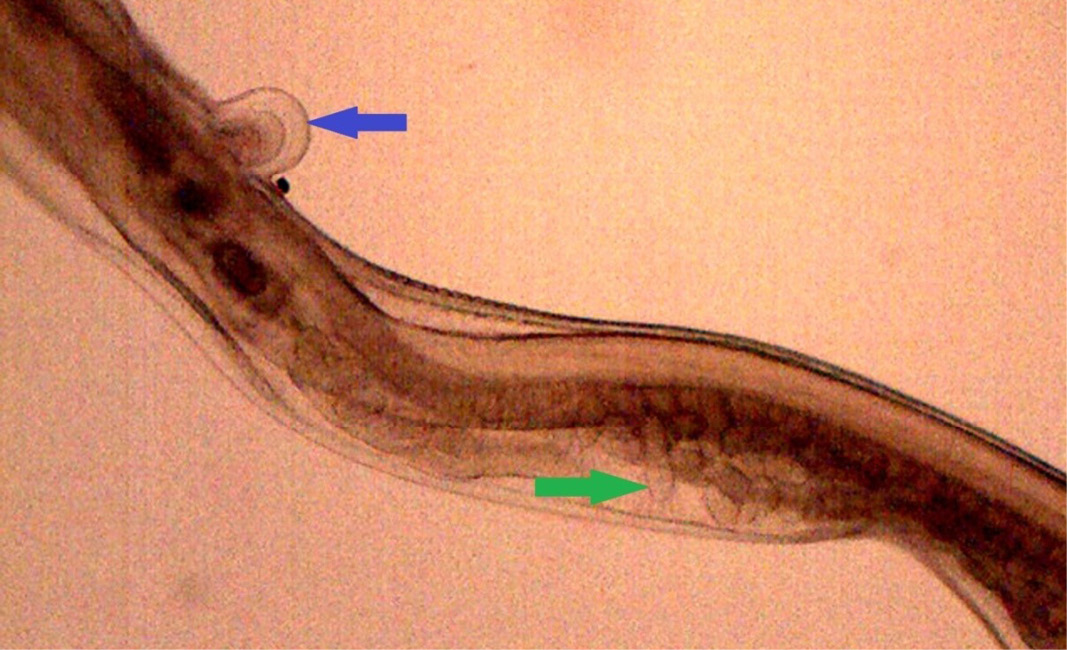
Figure 16: Posterior end of female H. contortus was characterized by knob type vulval flap (blue arrow) which covers the vulva and the uteri are fully packed with oval eggs (green arrow)
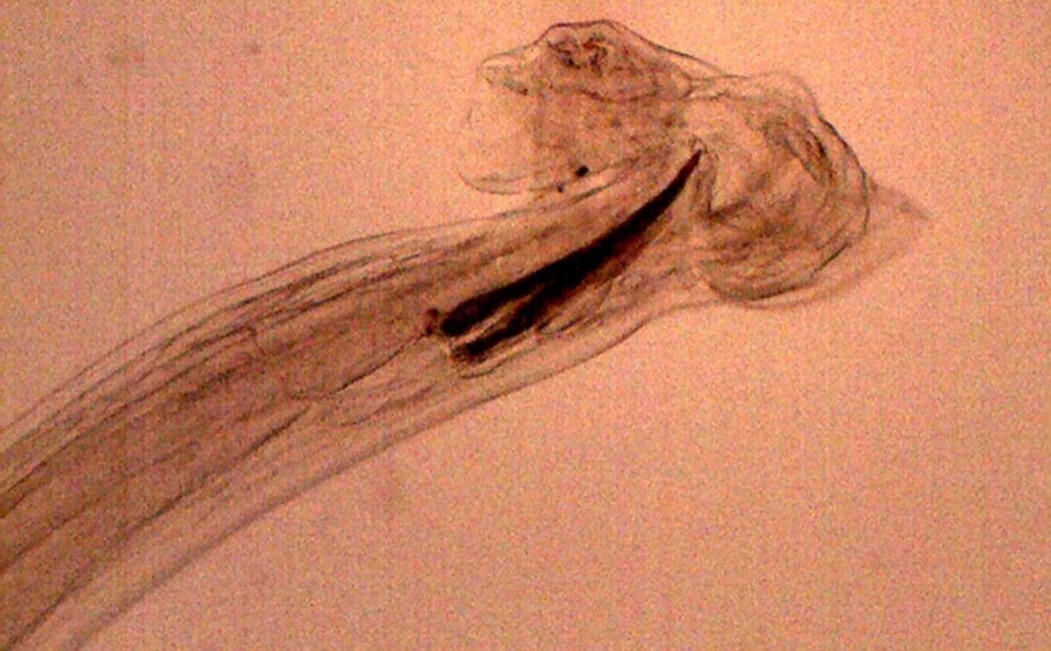
Figure 17: Posterior end of male H. contortus- copulatory bursa with well-developed asymmetrical dorsal lobes supported by inverted ‘Y’ shaped dorsal rays. Spicules are equal, short and end in knobs and are provided with barbs near the tip
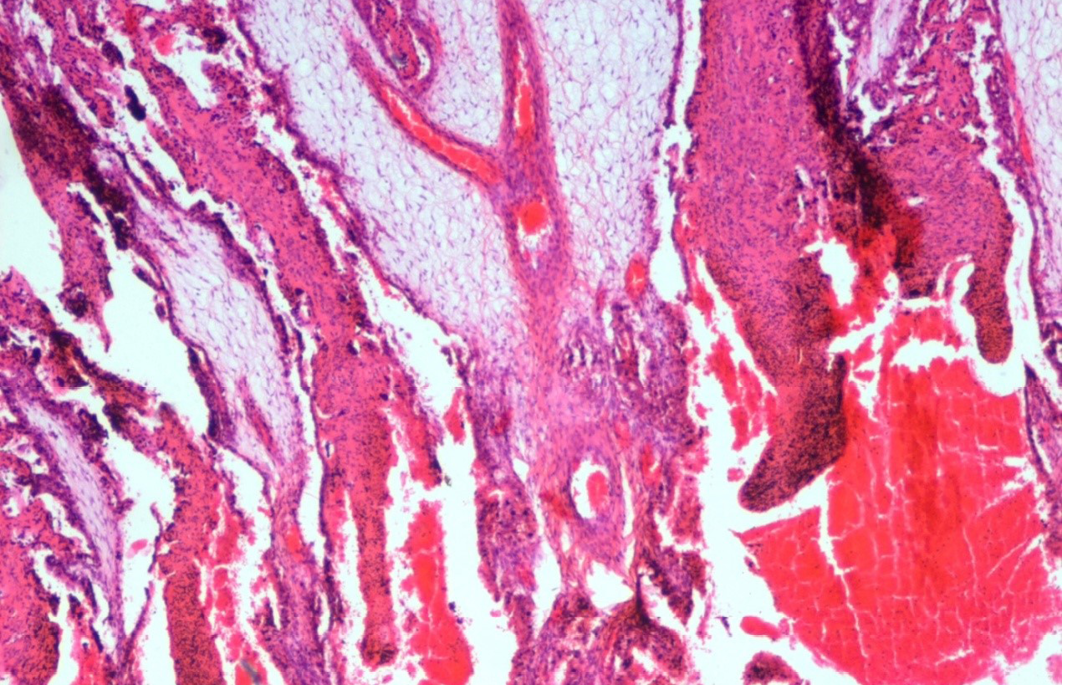
Figure 18: Abomasum showed haemorrhages, oedema, severe congestion of blood vessels in lamina propria and desquamation in the apical border of the villi
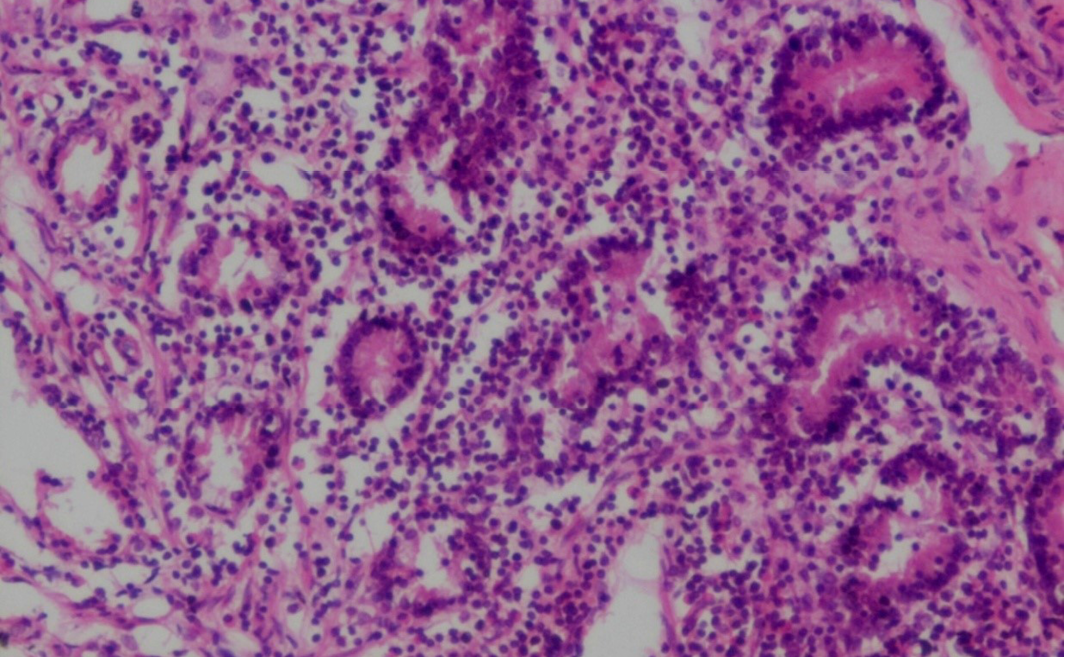
Figure 19: Prominent eosinophilic infiltration in the mucous and gastric glands of abomasum and infiltration of mononuclear cells especially lymphocytes
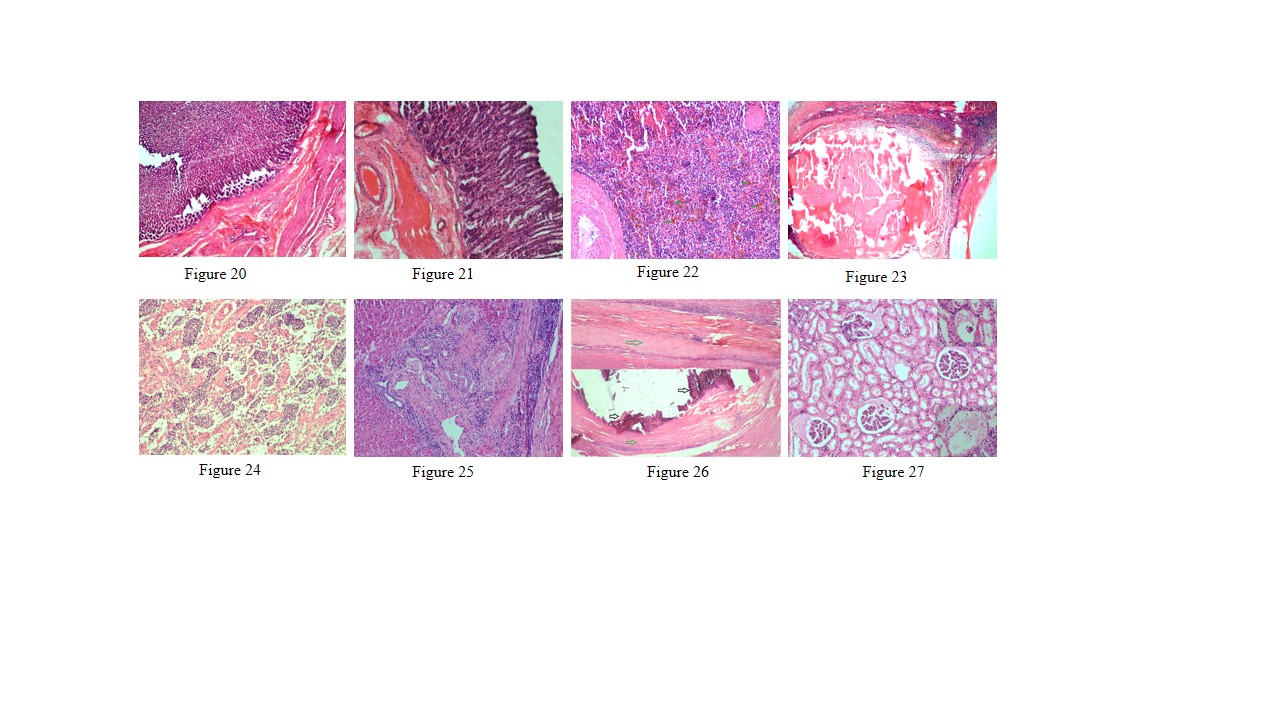
Figure 20: Thickening of abomasal mucosa due to hyperplasia of mucous and gastric glands
Figure 21: Small intestine showed highly congested capillaries in sub mucosa and thickened muscular layer
Figure 22: Spleen showed hemosiderosis (green arrows), depletion of red bulb area and prominent trabeculae
Figure 23: Spleen contains encapsulated cyst with homogenous eosinophilic material and cyst is lined by thick fibrous wall
Figure 24: Prescapular lymph node showed severe depletion of lymphocyte in both cortical and medullary region and prominent trabeculae
Figure 25: Liver showed mild bile duct hyperplasia, narrowing of bile duct lumen, degeneration of hepatocyte and mononuclear cell infiltration especially lymphocytes
Figure 26: Liver showed homogenous basophilic calcified mass (black arrows) which lined by thick fibrous capsule (green arrows)
Figure 27: Glomerulus showed eosinophilic protenacious edematous fluid and mild degenerative changes in the epithelium. Inset showing atrophy of the glomeruli
Numerous Haemonchus contortus eggs (+++) of thin-shelled, oval shape with equal poles, edges mombate and morula not fully filled the cavities of the eggs were identified (Figure 12). The eggs are approximately 70 – 85 µm in length and 41 - 48 µm in width. Few whip worm (Trichuris ovis) eggs (+) of dark brown colored, thick-walled, barrel shape with unsegmented embryo, no blastomeres and transparent polar plugs at both ends were found (Figure 13). Adult H. contortus worms were identified based on morphology (Soulsby, 1982). Male H. contortus worms are 10 to 20 mm long and are even reddish in colour. Female H. contortus worms are 18 to 30 mm long and the white uteri and ovaries winding around the red blood-filled intestine give a twisted or barber pole appearance (Figure 14). The anterior end of H. contortus was characterized by small funnel shaped buccal capsule, spine like pair of cervical papillae and transversely striated cuticle (Figure 15). Posterior end of female H. contortus was characterized by vulval flap which covers the vulva and it is small cuticular knob type (Figure 16). The posterior end of male H. contortus was characterized by copulatory bursa with well-developed asymmetrical dorsal lobes supported by inverted ‘Y’ shaped dorsal rays. Spicules are equal, short and end in knobs and are provided with barbs near the tip (Figure 17).
Lungs showed mild emphysema and cellular infiltration in inter alveolar septa. Heart showed separation and mild degeneration of cardiac muscle fibres due to edema. Abomasum showed hemorrhages, edema, severe congestion of blood vessels in lamina propria and desquamation in the apical border of abomasal villi (Figure 18). Prominent eosinophilic infiltration in the mucous and gastric glands of abomasum and some even penetrated to the sub mucosa, and infiltration of mononuclear cells especially lymphocytes (Figure 19). Thickening of abomasal mucosa due to hyperplasia of mucous glands was noticed (Figure 20). Small intestine showed highly congested capillaries in sub mucosa and thickened muscular layer (Figure 21). Apical border of caecal villi was desquamated, mononuclear cell infiltration and congested blood vessels were noticed. Spleen showed thick capsule, hemosiderosis, depletion of red bulb area and prominent trabeculae (Figure 22). Spleen also contains encapsulated cyst with homogenous eosinophilic material and cyst is lined by thick fibrous wall (Figure 23). Prescapular lymph node showed thick capsule, prominent trabeculae and depletion of lymphocyte in both cortical and medullary region (Figure 24). Liver showed congestion, mild bile duct hyperplasia, narrowing of bile duct lumen, degeneration of hepatocyte and mononuclear cell infiltration (Figure 25). Liver also showed homogenous basophilic calcified mass, lined by thick fibrous capsule (Figure 26). Glomerulus showed eosinophilic protenacious edematous fluid, atrophy of the glomeruli and mild infiltration of mononuclear cells (Figure 27). Tubular epithelium showed edema, mild degenerative changes and congestion of intertubular blood vessels. Mild edema was noticed in brain.
Haemonchus contortus is a major highly pathogenic and economically important gastrointestinal parasite of sheep and goats (Mortensen et al., 2003) and causes high morbidity, mortality, decreased production and economic loss due to cost of treatment and control measures (Githigia et al., 2001; Getachew et al., 2007; Qamar and Maqbool, 2012). In the study, gross lesions such as petechial haemorrhages due to the attachment and feeding of the parasite, and severe congestion in the abomasal mucosa were corresponded with the observation of McKenna (1998). In the present study, the carcass was pale, hide bound condition; gelatinization of subcutaneous tissue and mucous membrane were pale and anemic. These findings were corresponded with the observation of Courtney et al. (1984), Githigia et al. (2001), Zacharias et al. (2008) and Kelkele et al. (2012). The fluid was found throughout of the body cavities such as hydrothorax and hydroperitoneum (ascites) due to secondary tohypoproteinemia. Eosinophils responsible for pathogenesis during helminthic infection and are considered as first line of defence against parasitic infection, especially, haemonchosis. Eosinophilic infiltration was observed in the present study, which was agreement with the observation of Balic et al. (2000). The possible pathogenic mechanism responsible for cause of death in haemonchosis is hemorrhagic anaemia, hypoproteinemia and oedema of dependent parts due to vigorous blood sucking by both 4th stage larvae and adults (around 0.05 ml of whole blood/worm/day). To compensate the acute or chronic losses of plasma proteins and haemoglobin increased rate of RBC production occur results in depletion of iron stores leads to iron deficiency anaemia. In addition, diarrhoea results in fluid loss and dehydration leads to hypovolaemic shock (Scott et al., 1999; Mir et al., 2007; Zacharias et al., 2008; Kelkele et al., 2012). Liver showed degeneration of hepatocyte and mononuclear cell infiltration which has cause hypoproteinemia, anemia, and finally hypoxia condition.
Although fecal flotation examination is an important diagnostic tool to find out gastrointestinal parasitism, but H. contortus eggs cannot be distinguished easily from other parasites of strongylids family. In the present study, numerous H. contortus eggs (+++) and also few whip worm (Trichuris ovis) eggs (+) were identified on the basis of morphology (Soulsby, 1982). Adult female H. contortus worm was identified based on vulval flap and male was identified by spicules and copulatory bursa. These findings were corresponded with the observation of Valderrabano et al. (2002) and Kumsa et al. (2008).
CONCLUSION
Haemonchus contortus is an important blood-sucking nematode which has produce anemia and hypoproteinaemia in small ruminants that may be fatal particularly to young animals. Anemia is the major feature responsible for pathogenesis haemonchosis infection. The present study clearly describes the clinical signs, gross lesions of haemonchosis and their correlation with histopathological findings and parasitological examination. In the present study, cause of death due to specifically acute H. contortus infection results in hemorrhagic anaemia, hypoproteinemia, oedema of dependent parts, diarrhoea results in fluid loss and dehydration leads to hypovolaemic shock. The major activities for controlling haemonchosis infection includes rotational usage of different anti-helminthetic drugs, correct identification of parasitic species, correct usage of drug of choice, appropriate dosage of drug, change the grazing areas frequently to break the life cycle of parasite and rotational grazing methods.
REFERENCES