Journal of Animal Health and Production
Research Article
Experimental Trials of Spinal Cord Injury Treatment in Rats
Fathy Dosoky EL- Seddawy*, Mohamed Tayiser Mohamed Samy, Nefissa Helmy Mohamed Mekkawy, Ahmed El-Sayed Behery, Walaa Osama Mohamed Youssef
Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Zagazig University, 44511, Egypt.
Abstract | Severe spinal cord injury (SCI) is a global disorder in both human and animals. Current study investigated the role of platelets rich plasma (PRP) and bone marrow derived mesenchymal stem cells (BM-MSCs) in the treatment of acute compressive spinal cord injury in rat model. Twenty, non-pregnant female Sprague-Dawley rats about 6 months old with a weight of 250-300 g were subjected to laminectomy at the ninth thoracic vertebra (T9) followed by compression of the spinal cord using mosquito forceps for 15 seconds. Rats were equally divided into four groups; control group, PRP group, BM-MSCs group and PRP + BM-MSCs group. The rats were examined clinically at day 1, 7, 14, 21, and 28 postoperatively with Basso-Beattie-Bresnahan (BBB) scale, and then were sacrified after 28 days post operations for histopathological and immunohistochemical examinations. Gradual improvement of the locomotor function of the hind limbs with higher BBB scores were recorded in PRP and BM-MSCs treated groups in comparison to the control group. The use of PRP+BM-MSCs combination showed better white matter integrity and myelinated axons without glial scar formation than the use of either PRP or BM-MSCs alone and permanent pathological changes of the injured cord was shown in control group. The PRP+BM-MSCs combination showed poor immunogenic reaction for glial fibrillary acidic protein (GFAP) than all other groups and the control group showed higher immunoreactivity for GFAP with glial scar formation. It could be concluded that the locomotor activity of the hind limbs were improved after PRP and BM-MSCs treatment with limiting the pathological changes occurring after SCI.
Keywords | Spinal cord, Laminectomy, Platelets rich plasma, Rat, PRP, BM-BMSCs, GFAP
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | November 02, 2020; Accepted | November 20, 2020; Published | December 27, 2020
*Correspondence | Fathy D. EL- Seddawy, Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Zagazig University, 44511, Egypt; Email: [email protected]
Citation | EL-Seddawy FD, Samy MTM, Mekkawy NHM, Behery AE, Youssef WOM (2020). Experimental trials of spinal cord injury treatment in rats. J. Anim. Health Prod. 9(s1): 27-33.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/9.s1.27.33
ISSN | 2308-2801
Copyright © 2020 EL-Seddawy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Severe spinal cord injuries (SCIs) are one of the most challenging disorders in both human and veterinary health care practices (Šulla et al., 2018). Worldwide, SCIs incidence range from 13 to 163 per million people annually, according to the country (Kang et al., 2018). The SCIs are accounting for about 2% of all veterinary cases in dogs and cats (Olby and Jeffery, 2014). SCI is accompanied by neuronal degeneration and loss of the connections between the brain and other body systems, it leads to paralysis, disruption of sensory and autonomic functions and followed by serious complications (Šulla et al., 2018).
Different patterns of SCIs were recorded such as contusion, compression, hemisection, transection, and chemical injuries (Cheriyan et al., 2014). Compression and contusion injuries are the most commonly applied models for evaluation of the neuronal changes and behavioral outcomes in various lab animals.Compression model is a prolonged compression of the spinal cord for induction of a central hemorrhagic necrosis (McDonough et al., 2015; Sharif-Alhoseini et al., 2017).
Currently, SCIs appeared to be responsive for stem cells therapy where they provoke potential mechanisms like replacement of neuronal cells, re-myelination of axons and preservation of glial cells. Cell therapies depend on using different types of stem cells alone or in combination with other biomaterials or growth factors for induction of nerve regeneration or to neutralize the growth inhibitor factors (Dalamagkas et al., 2018l; Romero-Ramírez et al., 2020).
Bone marrow derived mesenchymal stem cells (BM-MSCs) are the most transplanted cells in tissue engineering as they have many advantages such as easy access, rapid in vivo expansion and poor immunogenicity. They promote motor function recovery and increase angiogenesis in the spinal cord after SCI in rats. They create a favorable environment for regeneration of the nervous tissues (Quertainmont et al., 2012; Romero-Ramírez et al., 2020).
Stem cells and Platelets rich plasma (PRP) combination therapies are become important issues and achieve wide expectations in regenerative medicine, particularly in tendon, cartilage and bone repair (Qian et al., 2017). Cho et al., 2010 proved that the combination between PRP and BMSCs has an effective role in regeneration of peripheral nerves as it acts as a scaffold and potentiates the effectiveness of BMSCs. Sariguney et al., 2008 proposed that cytokine receptors associated with PRP growth factors are present in the central nervous system on the surface of the neurons and glia cells.
The Basso, Beattie and Bresnahan (BBB) locomotor rating scale is an important test used to assess the behavioral outcomes following experimental SCI (Basso et al., 1995). Histological changes of injured spinal cord and immunohistochemistry of glial fibrillary acidic protein (GFAP) are important procedures for evaluating stem cells transplantation in rats (Al-Karim et al., 2019).
Spinal cord injury followed by devastating pathological changes causing permanent neurological deficits, therefore the aim of the present study was to investigate the role of PRP and BM-MSCs in treatment of acute compressive spinal cord injury in a rat model.
MATERIALS AND METHODS
Animals
Twenty female non-pregnant Sprague-Dawley rats about 6 months age with 250-300 g body weight were divided into 4 groups (5 rats in each group); control group, PRP group, BM-MSCs group and PRP+BM-MSCs group. All rats provided with a period of acclimatization for 2 weeks before the surgery. All procedures and the experimental design were approved by the Institutional Animal Care and Use Committee of Zagazig University (ZU-IACUC/2/F/139/2019).
Preparation and activation of PRP
Preparation of autogenic PRP was performed according to the previously described technique (Chen et al., 2018). Briefly, about 2mL citrated blood samples were collected from the rats of PRP and PRP+BM-MSCs groups in sterile centrifugation tubes through the medial canthus. The blood samples were double centrifuged at 220 g for 20min to and 480 g for 10 min to get PRP. The PRP was activated immediately before injection through addition of 50µL/ml of 10% CaCl2.
Preparation of BM-MSCs
Preparation and isolation of BM-MSCs were conducted in Stem Cells and Tissue Culture Laboratories, Medical Biochemistry and Molecular Biology Department, Faculty of Medicine, Zagazig University. Allogenic BM-MSCs were extracted from bone marrow of the femoral bones of male Sprague-Dawley rats using a modified method previously described (Osaka et al., 2010). Cells of second passage were used, were tested for viability by trypan blue test and were counted using the hemocytometer.
Surgery
Anesthesia was performed using I/M injection of ketamine (50mg/kg) and xylazine (5mg/kg) mixture and rats put in sternal position on a heating pad. Dorsal area between the neck and hind limbs was aseptically prepared.
The surgical approach of spinal cord injury was conducted similar to the technique of (McDonough et al., 2015) with modification and illustrated in Figure 1, all rats subjected to laminectomy at T9 and compression of the spinal cord with mosquito forceps for 15 sec. Treatment of injured spinal cord was performed according to group allocation and immediately after the compression: Control group, was injected with 0.1ml of saline solution; PRP group, was injected with 0.1ml of autogenic PRP; BM-MSCs group, was transplanted with 0.1ml of allogenic BM-MSCs suspension containing 1.5×106 cells; PRP+BM-MSCs group, was injected with 0.1ml of BM-MSCs suspension containing 1.5×106 cells and PRP mixture. The dose of therapeutic agent distributed intralesional, cranial and caudal to the injury site.
The operated rats were observed daily for clinical examination. The bladder was evacuated manually twice daily. Prophylactic administration of antibiotic (gentamycin 1mg/kg, q 12h/ 7 days) and analgesic (meloxicam 1mg/kg, q 24h/3 days) were performed.
BBB scoring-functional test
The BBB locomotion scale was used to evaluate the locomotor functional recovery of the two hind limbs after treatments of compressive spinal cord injury at days 1, 7, 14, 21, and 28 postoperatively. Two independent, blinded examiners observed each rat for 4 min. Hind limbs movements were recorded by video camera and locomotor functions were assessed from complete paralysis (score 0) to normal locomotion (score 21).
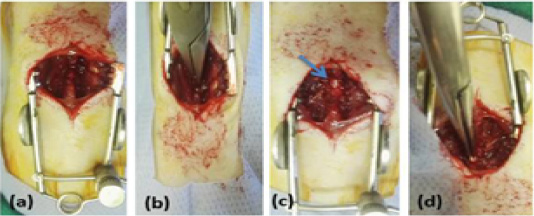
Figure 1: Showing surgical technique, (a) rat was in sternal position with sterilized dorsal area, longitudinal incision of skin and sharp incision of the muscles overlying the vertebral column T8–T11, (b) Laminectomy was done at T9, (c) exposing spinal cord (arrow), (d) Compression of the spinal cord with mosquito forceps for 15 sec.
Histopathological and Immunohistochemical examinations
All operated rats were sacrified after 28 days postoperatively three centimeters spinal cord samples at the injury site were collected, fixed in buffered neutral formalin, processed and embedded in paraffin wax. 4µm-thick sections were stained with hematoxylin and eosin examined using light microscope and photographed as described previously (Suvarna et al., 2018). For immunohistochemistry of glial fibrillary acidic protein (GFAP), the sections were deparaffinized and treated as described by (Kammerer et al., 2001).
Statistical analysis
Data of BBB scores were statistically analyzed with one-way ANOVA test. Duncan’s Multiple Range Test was applied to compare the significant differences among the means. Statistical analysis tests were performed by using version 17 of SPSS software 2008.
RESULTS AND DISCUSSION
Clinical findings
The urinary bladder regained its function spontaneously after operations within 7-10 days in control group, 5-7 days in PRP group, 3-5 days in BM-MSCs, and 2-3 days in PRP+BM-MSCs group.
Functional recovery of hind limbs
All the operated rats showed complete hind limbs paralysis immediately after injury that gradually improved and approach the level of complete recovery in PRP and BM-MSCs treated groups at the 28th day. The means ± SD of the BBB locomotion scores of all groups at days 1, 7, 14, 21 and 28 were recorded in Table 1 and illustrated with Figure 2. The BBB scores were significantly increased in all treated groups at all intervals in comparison to control group. The BBB scores of PRP group were significantly lower than BM-MSCs group and PRP+BM-MSCs group at 7, 14, 21 and 28 days of treatment. There was no significant difference between BM-MSCs group and PRP+BM-MSCs group at all intervals.
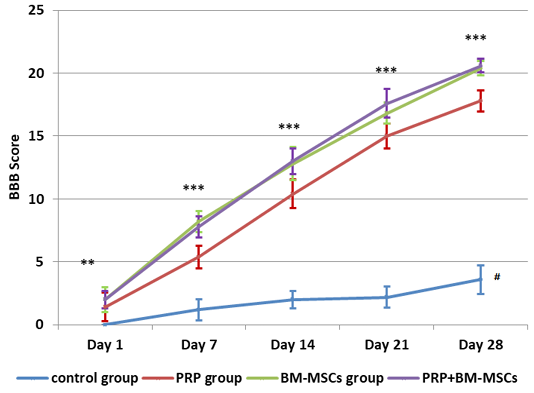
Figure 2: Showing BBB Score evaluation of hind limb locomotor recovery. # Lower significance than all other groups; ** Lower significance of PRP group than PRP+BM-MSCs group; *** Lower significance of PRP than BM-MSCs and PRP+BM-MSCs groups.
Histopathological findings
Sections of the control group revealed extended areas of cavitation surrounded by numerous reactive astrocytes with intensely acidophilic cytoplasm and dark shrunken nuclei (Figure 3A) and localized areas of hemorrhage and inflammation in other sections (Figure 3B). The PRP group showed enhancement of neural regeneration through induction of glial cells proliferation and control gliosis better than in control group, in addition to angiogenesis, and minor inflammation (Figure 3C). The BM-MSCs group showed reducing lesion cavitation and white matter loss and the grey matter showed control of gliosis or reduced astrocytes response with oligodendrocytes proliferation. The white matter integrity was better than the control and PRP groups, also BM-MSCs didn’t induce inflammatory reaction as PRP (Figure 3D). PRP+ BM-MSCs group revealed regulation of astrocytosis and improvement of white matter integrity, the sections showed proliferation of astrocytes, oligodendrocytes and microglia with myelinated axons (Figure 3E).
Immunohistochemistry
Immunohistochemistry was used for evaluation of the gliosis (reactive astrocytes) and glial scar formation in the injured spinal cord via GFAP expression. The control group showed down regulation of the GFAP at the site of the injury (compressed area) with high up regulation at the surrounding area due to increase the number of reactive astrocytes with thick cytoskeletal processes (marked gliosis), in addition to glial scar formation around the injury site (cranial and caudal to the lesion) (Figure 4A). PRP group showed less GFAP immunoreactivity than control group, but more than the other treated groups. PRP had a role in regulation of astrocytosis (GFAP expression) preventing occurring of the glial scar, there was moderate expression of GFAP cranial and caudal to the lesion with high expression centrally (Figure 4B). BM-MSCs group showed mild up regulation of GFAP expression in the cranial and caudal to the lesion with high regulation of GFAP expression at the site of the lesion (Figure 4C). PRP + BM-MSCs group showed well distribution of the GFAP expression and normal astrocytes distribution cranial and caudal to the lesion with mild GFAP expression and very few reactive astrocytes centrally at the site of the lesion (Figure 4D).
Spinal cord injury has become a major issue in modern regenerative medicine. Testing of new materials and different techniques that promote regeneration of the spinal cord tissue in animal models is necessary and very important during the preclinical stage because the recovery of spinal cord functions was hopeless (Gomes-Osman et al., 2016).
The selected compressive form of the spinal cord injury, it was depended mainly on the type which approved clinically as recoded in previous studies (Sharif-Alhoseini and Rahimi-Movaghar, 2014). Additionally, PRP and BM-MSCs was selected because of their roles in the regenerative medicine. PRP was a natural product prepared from the blood which have advantages like safe, available, ease of preparation, cheap and didn’t need expensive equipment in addition to releasing many growth factors have different benefits in nervous tissues regeneration as reported previously in spinal cord injuries in rats (Chen et al., 2018;Salarinia et al., 2017 and in peripheral nerve injuries (Kucuk et al., 2014).
On the other hand, advantages of BM-MSCs were clean accessibility, safe, and the ability for transplantation with hopeful therapy for SCI on different scientific studies (Othman, 2017). BM-MSCs enhanced neuronal protection and reduce the inflammation and glial reactivity. This was as mentioned by (Khan et al., 2018).
The usage of PRP and BMSCs together gave results better in spinal cord regeneration because PRP potentiates the efficacy of BMSCs treatments for SCI. this was in accordance to previous study for peripheral nerves regeneration (Cho et al., 2010).
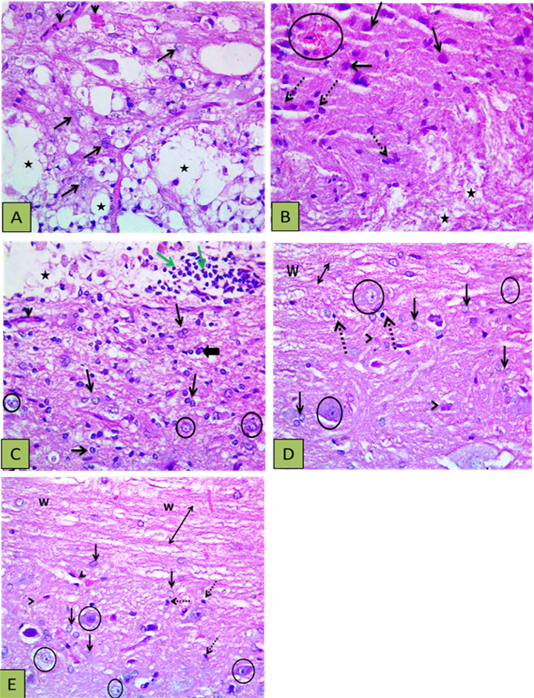
Figure 3: Showing histopathological findings. (A) Control group showing defective myelination (numerous areas of cavitation) (stars), reactive and increased size astocytes (arrows), and congested blood vessels (arrowheads); (B) Other section of control group showed the white matter with disrupted myelination (stars), the gray matter with numerous gemistocytic cells (reactive astrocytes shown by intact arrows), microglial cells proliferation (dashed arrows), and localized hemorrhage (circle); (C) PRP group showed astrocytic proliferation with increased size (reactive astrocytes) (thin arrows) oligodenrocytes (thick arrow), mild vascularity (arrowheads), with light stained intact neurons (circle) and white matter disruption (vacuoles shown by star) with inflammatory cells infiltration (green arrows); (D) BM-MSCs group showed intact axons (double heads arrow ) within white matter (w) and the grey matter showing astrocytic proliferation with increased size (blck arrows), oligodenrocytic proliferation (dashed arrow) minimal vascularity (arrowheads), with light stained intact neurons (circle); (E) PRP+BM-MSCs showing well myelinated nerve cells (upper side) (double heads arrow) within the white matter (w), astrocytic proliferation with increased size (intact arrows), microglia proliferation (dashed arrow) minimal vascularity (arrowheads), with light stained intact neurons (circle). H and E Photomicrograph with X400.
Table 1: Means and standard deviation of BBB scores.
|
Group Time point |
Control group Mean ± SD |
PRP group Mean ± SD |
BM-MSCs group Mean ± SD |
PRP+BM-MSCs Mean ± SD |
P- value |
| Day 1 |
0.000b ± 0.00 |
1.400a ± 1.14 |
2.000a ± 1.00 |
2.000a ± 0.71 |
0.005 |
| Day 7 |
1.200c ± 0.84 |
5.400b ± 0.89 |
8.200a ± 0.84 |
7.800a ± 0.84 |
0.000 |
| Day 14 |
2.000c ± 0.71 |
10.40b ± 1.14 |
12.80a ± 1.30 |
13.00a ± 1.00 |
0.000 |
| Day 21 |
2.200c ± 0.84 |
15.00b ± 1.00 |
16.80a ± 0.84 |
17.60a ± 1.14 |
0.000 |
| Day 28 |
3.600c ± 1.14 |
17.80b ± 0.84 |
20.40a ± 0.55 |
20.60a ± 0.55 |
0.000 |
Means in the same row bearing different letters a, b, c are significantly (p ˂0.005) different.
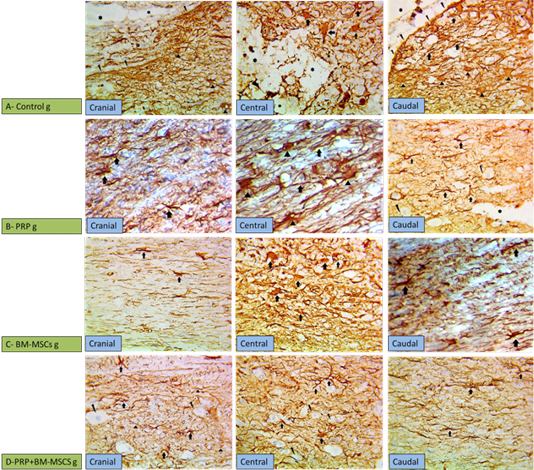
Figure 4: Showing immunohistochemical findings. (A) Control group showing wide injured area (star) in the gray matter with down regulation of GFAP at the center of the lesion with high expression of GFAP surrounding the lesion cranial and caudal to the lesion, noticed in the markedly hypertrophied astrocytes and their thickened processes (arrows), more thicker processes of astrocytes (arrow heads) denoting gliosis scar (broken arrows); (B) PRP group showing moderate expression of GFAP cranial and caudal to the lesion with marked gliosis centrally due to the increased number of astrocytes and their thickened processses (arrows); (C) BM-MSCs group, showing mild overexpression of GFAP cranial and caudal to the lesion with high overexpression (arrows) astrocytes due to thickening of their processes at the central area; (D) PRP+BM-MSCs group showing well distributed expression of GFAP denoting the normal expression with very few reactive astrocytes (arrows) in between neurons (broken arrows) cranial and caudal to the lesion and mild overexpression of GFAP at the central area of the lesion. (GFAP stain, x400).
In the present study, PRP was injected in the spinal parenchyma as an accurate method similar to method described previously Salarinia et al., 2017), while other method was intrathecal (Chen et al., 2018). Moreover, BM-MSCs were transplanted intralesional in the site of injury as described before (Othman, 2017). The intralesional injection prevented the loss of the cells which entrapted in other body systems after injection intravenous or intraperitoneal. This attribution was mentioned by (Muniswamia et al., 2019).
It was demonstrated that the outcome of BBB scores in the control group was unsatisfactory. All rats suffered from low scores of the hind limbs function at all the study period. Rats from the beginning to the end of the study exhibited hind limbs paralysis with sweeping during trying movement. These results were similar to the previously reported (Karaoz et al., 2012; Othman, 2017; Chen et al., 2018). Furthermore, Spinal cord injury especially traumatic injury leads to loss of function and paralysis of hind limbs (Sun et al., 2010).
The outcomes of the treated groups showed gradual improvement of locomotor function of the hind limbs better than the control one. Rats of PRP group were improved faster than the rats of control one and reached to higher scores as reported in previous studies Salarinia et al., 2017Chen et al., 2018). Rats in BM-MSCs group exhibited more improvement of locomotor function of the hind limbs than the rats of control and PRP groups (Karaoz et al., 2012; Othman, 2017). The treated compressed cord with combination of both PRP and BM-MSCs showed excellent recovery of the locomotor function of the hind limbs of all groups (Zhao et al., 2013).
Regarding to the histological and immunohistochemical examination, control group revealed permanent degenerative changes. These changes were similar to previously reported in rats by (Othman, 2017; AL-Karim et al., 2019).
Furthermore, histopathologiacal findings after PRP injection showed induction of astrocytes and microglia activation, angiogenesis, inflammatory response and had the ability to decrease area of cavitation and tissue loss better than control group. Similar results were reported previously (Chen et al., 2018). In BM-MSCs group, Transplantation of BM-MSCs was able to replace damaged spinal cord tissues and decrease areas of cavitation and hemorrhage which present in control group, also BM-MSCs lead to reducing glial scar formation through reducing the astrocytes response (Othman, 2017). The ability of BM- MSCs to form bridges through spinal cord cavity and reduce a glial scar led to axon growth and helped the tissue integrity, which was better than PRP group (Liu et al., 2019). Additionally, BM-MSCs promoted angiogenesis resulted in maintenance of adequate blood supply at the site of injury which was essential for neuroprotection (Carrion et al., 2013).
Moreover, combination between PRP and BMSCs had better results than when each used alone, because there was a synergistic effect on enhancing regeneration of compressed spinal cord. This was proved in regeneration of peripheral nerves study (Cho et al., 2010), because PRP act as scaffold potentiates the efficacy of BM-MSCs treatments for SCI.
Concerning to immunohistochemistry, control group showed reactive astrocytosis and confirmed by the highest level of GFAP expression surrounding the lesion with glial scar formation cranial and caudal to the center of the injury (Al-Karim et al., 2019).
The PRP injection activated the astrocytes and subsequent increased level of GFAP expression especially at the site of the injury. Similar results were reported previously in rats (Chen et al., 2018), while in BM-MSCs, transplantation of BM-MSCs showed few reactive astrocytes cranial and caudal to the center of the injury because the BM-MSCS had the ability to reduce astrocytes response. These results were similar to that found in rats by (Al-Karim et al., 2019).
Finally, in PRP+BM-MSCs group, the combination of PRP and BM-MSCs showed well distribution of astrocytes and normal GFAP expression cranial and caudal to the site of injury. It was approved that the expression of GFAP was decreased and the glial scar was reduced and axonal growth resulted. This attribution was mentioned before by (Yang et al., 2018).
Conclusions and Recommendations
The study revealed that rats with thoracic spinal cord compression showed complete paralysis of the hind limbs with degenerative histopathological changes indicating permanent neurological deficits. The treatment with PRP and/ or BM-MSCs resulted recovery of hind limb paralysis. Combination between PRP and BM-MSCs revealed better results for regeneration of spinal cord injury.
Author’s Contribution
All authors contribute to this research equally. They made available relevant literatures and participated in draft and revision of the manuscript. All authors read and approved the final manuscript. Surgical operations were done by Walaa Youssef.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






