Journal of Animal Health and Production
Short Communication
Comparative Assessment of Blood Pressure Parameters Between Different Sex and Different Reproductive Stages of Red Sokoto Goat
Bashir Saidu1*, Frank Babatunde Oluwole Mojiminiyi2, Clement Barikuma Innocent Alawa3, Chinedu Onwuchekwa2, Murtala Bello Abubakar2, Umar Zayyanu Usman2, Adamu Jibril Bamaiyi2, Kasimu Ghandi Ibrahim2
1Department of Physiology and biochemistry, Faculty of Veterinary Medicine, Usmanu danfodiyo University Sokoto, Nigeria; 2Department of Physiology, College of Health Sciences Usmanu danfodiyo University Sokoto, Nigeria; 3Department of Animal production, Faculty of Veterinary Medicine, University of Abuja, Nigeria; 4Department of Public health and preventive medicine, Faculty of Veterinary Medicine, Usmanu danfodiyo University Sokoto, Nigeria.
Abstract | Extensive system of management and physiological changes at different reproductive stages causes changes in circulatory functions which could affect blood pressure parameters. The study was conducted to establish the normal blood pressure of red Sokoto goat and to compare between sex and reproductive stages. The study was conducted using Thirty Red Sokoto goats (RSG) comprising of fifteen bucks and fifteen does with mean age of 2±0.17 years and average weight of 35±0.5 kg. The goats were synchronised using CIDR(R) and were naturally mate. Bright (B) mode real time ultrasound scanning was conducted using 2.5 MHz sector transcutaneous probe to establish pregnancy. Blood pressure parameters (Systolic, Diastolic, Mean arterial pressure and pulse rate) were measured using electronic sphygmomanometer (CONTEC08A-VET) and student t-test and ANOVA were used to compare between the sex and reproductive stages respectively. Red Sokoto bucks have significantly higher (P˂0.05) blood pressure parameters compared to red Sokoto does, but there was no significant (P˂0.05) difference between the reproductive stages. Red Sokoto bucks have higher blood pressure parameters compared to Red Sokoto does. However, reproductive stages have no effect on blood pressure parameters of Red Sokoto goat does.
Keywords | Comparative, Assessment, Blood pressure, Red Sokoto goat, Reproductive, Stages
Received | June 29, 2020; Accepted | July 27, 2020; Published | October 05, 2020
*Correspondence | Bashir Saidu, Department of Physiology and biochemistry, Faculty of Veterinary Medicine, Usmanu danfodiyo University Sokoto, Nigeria; Email: [email protected]
Citation | Saidu B, Mojiminiyi FBO, Alawa CBI, Onwuchekwa C, Abubakar MB, Usman UZ, Bamaiyi AJ, Ibrahim KG (2020). Comparative assessment of blood pressure parameters between different sex and different reproductive stages of red sokoto goat. J. Anim. Health Prod. 8(4): 189-192.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/8.4.189.192
ISSN | 2308-2801
Copyright © 2020 Saidu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Goats are among the oldest domesticated animal species, they are greatly adaptable to the tropical regions of the world (Hooda and Upadhyay, 2014). The northern region of Nigeria plays an important role in goat production in the African continent and the world in general (Njidda et al., 2013). Goat forms an integral part of the economy of most rural dwellers in northern Nigeria as they are found in almost all households and are kept as means of savings and sold to raise money to cater for emergency situations (Abdalla et al., 2009). These rural dwellers mostly practice extensive system of management which expose the animals to several factors capable of causing blood pressure changes. Goat exhibits diversified physiologic ability due to their ability to adapt to different environmental conditions.The importance of the cardiovascular system is the reason why many studies on identification and treatment of cardiac diseases have been carried out using different domestic animal models. Pregnancy and lactation are physiological processes that cause hormonal, electrolyte and cardiovascular changes that affect different systems of the animal which could result in blood pressure changes. Despite the resilience of red Sokoto goat, no documented literature on its blood pressure parameters is available. The ability of goat to adapt to different environmental condition may not be unconnected with circulatory sufficiency, but little consideration is given to blood pressure parameters of goats which hides the potential of using goat as a model for cardiovascular researches. The study is aimed at establishing a base line data of blood pressure parameters of red Sokoto goat and to determine the relationship between sex and blood pressure parameters.
Materials and methods
A total of thirty goats comprising of fifteen bucks and fifteen does with a mean age of 2.0 ± 0.7 and average weight of 35.0 ± 0.5 kg divided in to group 1 (Bucks) and group 2 (Does) were used for the study. Wheat bran and bean husk was fed and water was provided ad libitum.
Research Design
Ethical approval was obtained from Faculty Animal Research Ethics Committee, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto (UDUS/FAREC/2017/AUP-RO-06)
The research was divided in to two phases.
Phase one: The animals were conditioned for two weeks, assessed for pregnancy using ultrasonography and normal blood pressure parameters were established.
Phase two: The period involved oestrous synchronisation using control internal drug release (CIDR)(R), mating of the animals, assessment for pregnancy using ultrasonography and recording of blood pressure parameters.
Oestrous Synchronisation
Oestrous synchronisation was conducted using controlled internal drug release (CIDR(R)) as described by (Islam, 2011). The animal was restrained in a standing position with the tail raised and the perineum was wiped with a paper towel. The CIDR(R) was lubricated using KY jelly, a normal cellulose lubricant. The lateral commeasures of the vulva were gaped, the mounted applicator was inserted and the plunger of the applicator was pressed to release the CIDR(R) into the vagina. The CIDR(R) was removed three weeks after the insertion and the animals were monitored for signs of oestrous.
Observation of Oestrous
The CIDR(R) was removed three weeks after insertion and the animals were monitored for signs of oestrous. There was swelling of the vulva, mucoid discharge from the vulva and standing heat was also observed. A buck was introduced and natural mating was allowed. After four weeks of mating, the does were assessed for pregnancy using ultrasonography as described by (Streeter, 2007).
Pregnancy Assessment
As a preparation for ultrasonography, the animals were fasted overnight by withdrawing feed. Ultrasonography was conducted as described by (Streeter, 2007). The inguinal region was shaved and cleaned with chlorhexidine. The animal was placed in a standing position and acoustic gel was applied on the shaved area. Bright (B) mode real time ultrasound scanning was conducted using 2.5 MHz sector transcutaneous probe of Vet image 201 ultrasonography machine of Recorders and Medicare Systems (RMS) (P) LTD (Panchkula Haryana, India).
Blood Pressure Monitoring
The blood pressure was measured based on Oscillometric method using electronic sphygmomanometer (CONTEC08A-VET) as earlier described by (Lee, 2013). The animal was placed on standing position and the cuff was placed around the humerus. The animal was allowed to stand for 30 minutes and the device was put on. The values of Systolic pressure, Diastolic pressure, Mean arterial pressure (MAP) and Pulse rate were displayed on the screen. Blood pressure was monitored before pregnancy, during pregnancy and after pregnancy.
Statistical Analysis
Data generated was analysed using SPSS version 20. Student t-test was used to compare between the sexes while analysis of variance (ANOVA) was used to compare between the periods.
Results and discussion
The blood pressure of the Red Sokoto bucks and does are presented in Figure 1. Comparison of the various blood pressure components revealed significantly (P˂0.05) higher systolic pressure in red Sokoto bucks than in red Sokoto does. There was also significantly (P˂0.05) higher diastolic pressure in red Sokoto bucks than in red Sokoto does. Similarly, mean arterial pressure was also significantly (P˂0.05) higher in red Sokoto bucks than in red Sokoto does. In addition, pulse rate was significantly (P˂0.05) higher in red Sokoto bucks than in red Sokoto does. However, comparison of Blood pressure parameters (Systolic pressure, Diastolic pressure, Mean arterial pressure and Pulse rate) of red Sokoto goats before pregnancy, during pregnancy and after pregnancy revealed no significant difference as presented in Figure 2.
To the best of our knowledge this is the first documented report of blood pressure of Red Sokoto goat. The significantly (P˂0.05) higher Blood pressure parameters in the bucks compared to does could be attributed to the difference in testosterone level between male and female. This hormone enhances response to stimulation of resistance
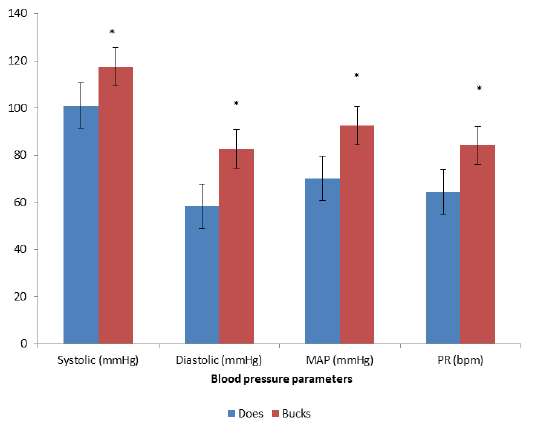
Figure 1: Systolic Pressure, Diastolic Pressure, Mean Arterial Pressure (MAP) and Pulse Rate of Red Sokoto Bucks and Red Sokoto Does
MAP = Mean Arterial Pressure
PR = Pulse Rate
* = P<0.05 bucks vs does

Figure 2: Systolic Pressure, Diastolic Pressure, Mean Arterial Pressure (MAP) and Pulse Rate of Red Sokoto Does at different reproductive stages
MAP = Mean Arterial Pressure
PR = Pulse Rate
vessels by increasing α adrenergic receptors in the smooth muscle of the blood vessels (Liu and Ely, 2011). It also enhances the release of norepinephrine (Liu and Ely, 2011). It could also be due to its effect in inhibiting the degradation of norepinephrine which increases its availability to bind to its receptor thereby causing vasoconstriction (Dalmasso, 2017). It could also be due to its effects on the renin-angiotensin system, this is because testosterone has receptors in the proximal convoluted tubule thereby enhancing sodium reabsorption and production of vasoconstrictor substances (Maric-Bilkan and Manigrasso, 2012).
The absence of significant (P˂0.05) difference between the reproductive stages could be attributed to the variation in the level of different hormones at different reproductive stages. Estrogen is predominant in non-pregnant animals and it stimulates and enhances the synthesis and release of Nitric oxide (NO) (Vitale et al., 2010). It also results in coronary artery relaxation by stimulating the closure of calcium channels (Liu and Ely, 2011). Progesterone level is elevated during pregnancy and it has receptors on the myocardium and affects the long lasting calcium channels that maintained electrical transmission in the heart (Lin et al., 1982). It could also be attributed to the decreasing effect of progesterone on membrane calcium concentration of endothelial cells of blood vessels (Barbagallo et al., 2001). This reduces blood pressure through enhancing vasodilation (Nicholson, 2017). The absence of significant (P˂0.05) increase in blood pressure after pregnancy compared to the periods before pregnancy and during pregnancy could be attributed to the increased levels of Oxytocin and Prolactin. Prolactin receptors are present on the endothelial lining of larger blood vessels and causes increase in intracellular calcium and a consequent increase in Nitric oxide production resulting in vasodilation (Gonzalez et al., 2015). Oxytocin on the other hand stimulates the release of atrial natriuretic peptide which results in decreased cardiac contractility and slows the heart rate (Gutkowska et al., 2014;; Charkoudian et al., 2017).
Conclusion
Red Sokoto bucks have higher blood pressure than red Sokoto does, while reproductive activities have no effect on blood pressure parameters of red Sokoto does.
Acknowledgement
The authors acknowledge the contribution of Yusuf Yakubu of the department of Veterinary Public Health and Preventive medicine, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto.
Conflict of interest
The author wish to declare that they had no conflict of interest
authors contribution
All authors contributed equally.
References






