The Journal of Advances in Parasitology
Research Article
Locally Transmitted Malaria Focus in Aswan Governorate, Upper Egypt
Asmaa El-Kady1, Osama Hussein Abdell1, Amal Mostafa2, Salah Mohammed Hussein3
1Department of Medical Parasitology, Faculty of Medicine, South Valley University; 2Department of Medical Parasitology, Faculty of Medicine, Sohag University; 3Department of Medical Parasitology, Faculty of Medicine, Al Azhar University, Egypt.
Abstract | Re-emerging malaria is a major health problem in many countries. Methods: The present study was conducted on a total of 37 cases to diagnose malaria cases during an outbreak of malaria in Edfo district, Aswan governorate, Egypt in the period from 26th May to 28th June 2014. Patients were subjected to clinical history taking, laboratory examination (thin and thick blood films) for detection of different malarial stages was done. Data was collected, and analyzed using SPSS software version 16 under windows 7. Results : Microscopic examination of blood films of the 37 cases revealed the presence of Plasmodium stages in 23 patients (62.2%). Different stages of plasmodium vivax were detected in 22 patients using thick and thin blood film while ring stages and gametocytes of Plasmodium falciparum were detected by thick blood film examination in one of the patients. Conclusion: Although it is difficult to have a significant outbreaks of malaria in Egypt, the risk of localized outbreaks of introduced cases is present due to infection of local anopheline mosquitoes by imported cases as many anophyline spp. which serve as malaria vectors are present in Egypt.
Keywords | Locally transmitted, Malaria, Upper Egypt, Aswan, Governorate.
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | January 21, 2017; Accepted | February 24, 2017; Published | February 28, 2017
*Correspondence | Asmaa El-Kady, Department of Medical Parasitology, South Valley University, Egypt; Email: [email protected]
Citation | El-Kady A, Abdell OH, Mostafa A, Hussein SM (2017). Locally transmitted malaria focus in aswan governorate, upper egypt. J. Adv. Parasitol. 4(1): 23-27.
DOI | http://dx.doi.org/10.17582/journal.jap/2017/4.1.23.27
ISSN | 2311-4096
Copyright © 2017 El-Kady et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Malaria as a disease has been known in Egypt since ancient times (Massa et al., 2000). Malaria was endemic in almost all Governorates of the country but prevalence showed a marked decline by 1990, and regressedin most of the Governorates (WHO, 2004). Egypt was classified within group 2 of the EMR countries where malaria was firmly under control and aiming at eradication of the disease (World Malaria Report 2013). During 2010–2013, Egypt successfully eliminated malaria (maintained zero indigenous cases), but remained in the stage of prevention of re-introduction of malaria. (World Malaria Report, 2016)
In fact, the country had reports of introduced cases or limited outbreaks of indigenous cases (Kenawy, 2015). The last focus of malaria in Egypt was in El-Fayoum after which Egypt is free of internally acquired cases of malaria since 1998 (WHO, 2012). As all the recorded cases were imported mainly from Sudan (WHO, 2012). Re-emerging malaria often becomes a serious public health problem in many countries (Sharma, 1996).
Materials and methods
The present study is a retrospective study was conducted on a total of 37 cases from Aswan Governorate during the period from 26 May to 28 June 2014. Patients were presenting with symptoms suggestive of malaria as fever, rigors, headache or darkening of urine.
Blood samples were collected from all cases included in the study. Samples were collected by sterile syringes then evacuated in EDTA containing blood collecting tubes labeled with patient name and date of collection. Thin and thick blood films were immediately prepared and stained.
All cases incorporated into the present work were subjected to history taking including individual history: name, age, and residence, history of voyaging abroad, Present history (manifestations): rigors, fever, sweating, headache and darkening of urine and general examination for paleness and jaundice and abdominal examination for discovery of splenomegaly.
Thin and thick blood films examination for detection of different stages of malaria was done. Thin blood films were prepared according to (Cheesbrough, 1999), where a small drop of blood is placed near the frosted end of a clean glass slide. A second slide is used as a spreader,placing the spreader at a 45° angle and backing into the drop of bloodthe blood is streaked in a thin film over the slide. The slide is allowed to air-dry and is thensmears were air-dried, and then dipped into 100% methanol, we place a layer of stain in the bottom of a glass jar (about 3 mL), then add buffer to a level that will just cover the slides when they are in the jar. Stain at room temperature for about 20 minutes. Then remove slides, rinse by dipping a few times into plain buffer, then stand on end to dry. Blood films were then allowed to air-dry in a vertical position and examined under a light microscope X100.
Thick blood films were prepared according to (Salak et al., 1999) where a drop of blood was placed at the a clear end and use the edge of the smearing slide to spread the drop out to about a 1 cm circle. The thick smear will take longer to dry without fixative then the spot was stained with diluted Giemsa (1:20, vol/vol)for 20 min., the slides was washed by placing the film in buffered water for 3 min., then blood films were allowed to air-dry in a vertical position and were examined using a light microscope X100.
Statistical Analysis
Data was collected and data analysis was performed using SPSS software version 16 under windows 7.
Results
The total number of cases involved in the study was 37 cases. Their ages ranged from 2.5 to 90 year with mean of 23.76, median 19 and standard deviation of 18.75. Outof all cases, 23 cases were positive for malaria as shown in (Table 1).
As regards gender among the positive cases, 11 (47.8%) were males and 12 were females (52.2%) as shown in (Table 2).
Out of 23 positive cases , all cases had elevated body temperature (100 %) ,11 person (47.8 %) had rigors, 12 persons (52.2%) had headache ,1 person (4.3 %) had tachypnea ,4 persons (14.4%) had darkening of urine, 2 persons (8.6 %) had tachycardia with no cerebral coma (0%) as shown in (Table 3). It was found that 4 persons (14.4 %) had splenomegaly, and 23 persons (100 %) had palloras illustrated in (Table 3).
Table 1: Showing the result of examined cases.
|
Result |
N |
% |
|
Negative |
14 |
37.8 |
|
P . falciparum |
1 |
2.7 |
|
P . vivax |
22 |
59.5 |
|
Total |
37 |
100.0 |
Table 2: Gender distribution among positive cases.
|
Diagnosis |
|||||||
|
P. falciparum |
P. vivax |
Total |
|||||
|
N |
% |
N |
% |
N |
% |
||
|
Sex |
Male |
1 |
4.4 |
11 |
47.8 |
12 |
52.2 |
|
Female |
0 |
0 |
11 |
47.8 |
11 |
47.8 |
|
|
Total |
1 |
4.4 |
22 |
95.6 |
23 |
100 |
|
Microscopic examination of blood films of the 37 cases revealed presence of Plasmodium stages in 23 patients (62.2%). Different stages of plasmodium vivax was detected in 22 patients using thick and thin blood film while ring stages and gametocytes of Plasmodium falciparum were detected by thick blood film of one case as shown in the following (Figure 1, 2, 3, 4, 5).
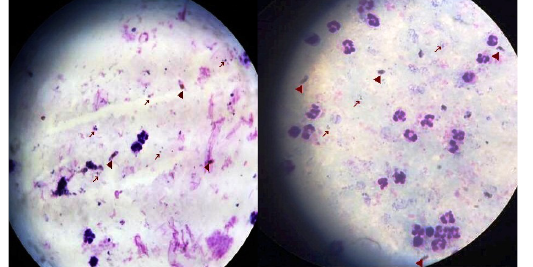
Figure 1: Thick blood film stained with Lieshman stain showing Plasmodium falciparum ring stages with magnification power 100x.

Figure 2: Thick blood film stained with Lieshman stain showing Plasmodium falciparum ring (long arrows) and gametocytes stages(short arrows) with magnification power 100x
Table 3: Showing distribution of symptoms and signs among cases
|
Presentation Diagnosis |
Anemia |
Fever |
Rigors |
Headache |
Splenomegaly |
Jaundice |
Tachypnea |
Tachycardia |
|
P.falciparum |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
P. vivax |
22 |
22 |
10 |
11 |
3 |
3 |
0 |
1 |
|
P. value |
0.000 |
N.C |
0.286 |
0.533 |
0.026 |
0.026 |
0.001 |
0.000 |

Figure 3: Thick blood film stained with Lieshman stain showing Plasmodium vivax ring and trophozoites stages with magnification power 100x
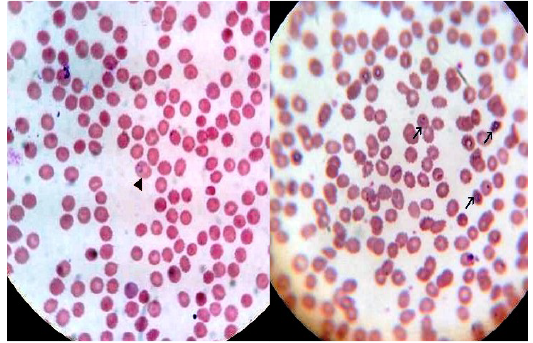
Figure 4: Thin blood film stained with Lieshman stain showing Plasmodium vivax ring stages with magnification power 100x
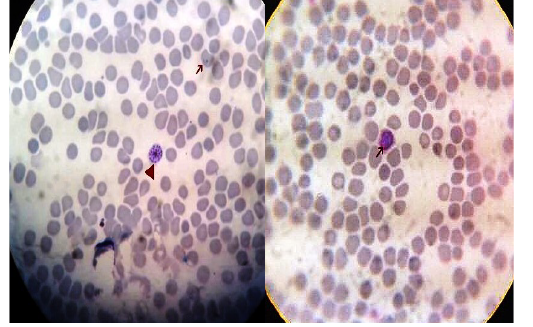
Figure 5: Thin blood film stained with Lieshman stain showing Plasmodium vivax gametocytes (long arrows) and shizonts (short arrows) with magnification power 100x
Discussion
The condition started on 26thMay 2014when a male patient aging 37 years from El Sheikh Mostafa village, Edfo District, Aswan Governorate, complained of fever. Hewas admitted to Edfo Fever Hospital and malaria was confirmed with peripheral blood examination which showed Plasmodium vivax infection.
Subsequent cases were discovered according to the clinical picture of patients which mainly include fever and darkening of urine. House to house survey was helpful in discovery of cases.
The total number of suspected patients (on basis of clinical presentation) was 38 cases. The number of confirmed cases was 23 patients, 22 were diagnosed as P. vivax and one old lady with P. falciparum infection,with equal rate of transmission in males and females where 11 were males and 12 females, individuals of all age categories were affected.
In the present study, fever was a symptom in all malaria positive patients and this agreed with several studies screening for the common clinical presentation of malaria (D’Acremont, 2010), and also in accordance with (Genton and D’ Acremont , 2001) who stated that most of malaria patients experience fever (>92%), chills (79%) and headaches (70%) . In addition, (Yaw et al., 2014) stated that in non-endemic areas, peripheral parasitaemia accompanied by fever could be used to define clinical malaria.
On the other hand, (Murray et al., 2007), stated that fever is not always a feature of malaria, and signs may be unusual with effective prophylaxis of malaria. Also, (Sakaria et al., 2013) reported that the usual presentation of malaria with febrile attacks is seen only in 50-70% of the patients.
None of the cases gave a history of travelling abroad to any of the neighboring malarious areas which suggests that the A swan outbreak of malaria was introduced following an imported case or cases from neighboring malarious areas followed by local transmission. A less likely source of the epidemic would have been relapse or recrudescence of P. vivax, as none ofthe patients reported prior history of malaria.
The diagnosis of cases was plasmodium vivax in 22 cases (95.7%) and only one old aged female with plasmodium falciparum infection representing (4.3%).
Patients received treatment in the form of a combination of (20 mg artemether and 120 mglumefantrine), twice daily with for 3 days ,then single dose of Primaquine (0.25 mg⁄ kg) was administered fromthe fourth day onwards until the 14th day, and after the blood film became negative for malaria,. The P. falciparum infected case was treated similarly.
Peripheral blood film examination was made daily for 3 consecutive days after treatment, and patients were not discharged until their blood films are negative for plasmodium malaria.
Reported cases of malaria in Egypt in the period from 1998 – 2010 were all imported as reported by (WHO, 2012; Daoud, 2003) about the distribution of malaria cases in Egypt reported by the Ministry of Health from 1998 – 2010 as illustrated in (Table 4 and 5).
Although it is difficult to have a significant outbreaks of malaria in Egypt the risk of localized outbreaks of introduced cases is present due to infection of local anopheline mosquitoes by imported cases as many anophyline spp. which serve as malaria vectors are present in Egypt as Anophyles pharoensis and Anophyles sergenti are the detected vectors in Egypt. Anophyles pharoensis is for the most part the vector of Plasmodium vivax while, An. sergenti is the vector of P. falcipaum (Wassim, 2014).
Table 4: Recorded Imported Malaria cases 1998-2003 in Egypt (Daoud, 2003).
|
Year |
No of examined slides |
Positive |
P. vivax |
P. falciparum |
|
1998 |
32403 |
24 |
23 |
1 |
|
1999 |
28992 |
38 |
37 |
1 |
|
2000 |
26581 |
17 |
17 |
0 |
|
2001 |
26341 |
10 |
9 |
1 |
|
2002 |
25785 |
9 |
9 |
0 |
|
2003 |
23813 |
45 |
44 |
1 |
Table 5: Malaria in Egypt from 2004-2010 (WHO, 2012)
|
Year |
Suspected cases |
Examined microscopically |
Confirmed microscopically |
Imported cases |
|
2004 |
43 |
43 |
43 |
43 |
|
2005 |
23 |
23 |
23 |
|
|
2006 |
29 |
29 |
29 |
|
|
2007 |
30 |
23402 |
30 |
30 |
|
2008 |
80 |
34880 |
80 |
80 |
|
2009 |
94 |
41344 |
94 |
94 |
|
2010 |
85 |
664294 |
85 |
85 |
Several factors increase the risk of reemerging malaria in Egypt including the continuous movement of people between Egypt and Sudan through Aswan governorate and the entrance of Asian and African peoples to Egypt for different purposes including educational and touristic purposes and anti-malarial drug resistance (Thomas and Nchinda, 1998). Other factors as water resources development projects (e.g. Toshka and El Salam Canal) also increases the risk. Several studies had linked environmental change caused by these projects and risk of arthropod-borne disease (Birley 1989).
Re-introduction of malaria has occurred in some Eastern Mediterranean Region countries that have been free of malaria. For instance, an imported case from Pakistan resulted in a small outbreak (30 cases of vivax malaria) in Jordan during 1990. In 1998, due to Somali refugees, a small outbreak (21 cases of falciparum malaria) occurred in Oman which had been free of falciparum malaria (but not vivax). Also, in Lebanon 2 small outbreaks of vivax malaria occurred during 1997–1998 (WHO, 2007).
In the European Region, local transmission from imported cases has been reported in Republic of Moldova (2003), Ukraine (2003), France (2006, 2008–2010), Italy (2007), Greece (2010) three locally transmitted cases were recorded, and Spain (2010).In all these instances, local outbreaks were limited to fewer than 10 cases (WHO, 2012)
In other parts of the world: three cases arising from local P. falciparum transmission were reported in Singapore in 2003; Oman, which interrupted transmission in 2004, has experienced several subsequent outbreaks of P. vivax and P. falciparum brought in by migrant workers from the Indian subcontinent; and Morocco, certified malaria-free in 2007, recorded two cases of “airport malaria” in 2009 (WHO, 2011).
AuTHOR’S CONTRIBUTION
Idea by Asmaa EL Kady, Osama Abdella. Asmaa EL Kady performed the laboratory works and collected data, Amal Mostafa and Salah Mohammed Hassan helped with the laboratory analysis of samples, collection of papers, data analysis and writing the manuscript and Osama Abdella , Asmaa EL Kady revised the manuscript.
Conflict of interest
We (the authors) declare that we all have no conflict of interest.
ACKNOWLEDGEMENTS
Our special acknowledgment to Dr. Magda El-Nazer, Professor of Medical Parasitology, Faulty of Medicine, Assuit University who helped us exammining the blood films of the patients.
Ethics Approval
This study was approved by the Institutional Ethics Review Board of the Faculty of Medicine, South Valley University, Qena, Egypt. Oral consent was obtained from the patients before they were recruited into the study.
References
Featuring
-
Prevalence of Gastrointestinal Parasites in Sonali Chicken at Sadar Upazila of Dinajpur
Md. Gausur Rahman, S.M. Harun-Ur-Rashid, Md. Golam Azam, Md. Ahsan Habib, Golapi Rani Devsharma
J. Adv. Parasitol., Vol. 11, pp. 14-18.
-
Seasonality of Neglected Tropical Geohelminthes and Asymptomatic Pfalciparum Malaria Prevalence Among Pregnant Women Attending Antenatal Care at NandiHills Subcounty Hospital Kenya
Rael Jepkogei Masai
J. Adv. Parasitol., Vol. 11, pp. 5-13
-
Bed Bugs Infestation and Control at a Tea Stall in Lahore City: A Case Study
Kashif Nazir, Asmat Nawaz, Shahzad Hur, Mashal Mehreen, Moazam Ali Saim, Seraj Ud Din, Inam Ul Haq
J. Adv. Parasitol.., Vol. 11, pp.1-4
Subscribe Today
Receive free updates on new articles, opportunities and benefits

© 2024 ResearchersLinks. All rights Reserved. ResearchersLinks is a member of CrossRef, CrossMark, iThenticate.





