Advances in Animal and Veterinary Sciences
Research Article
In vitro Efficacy of Verticillium lecanii and Beauveria bassiana of Commercial Source Against Cattle Tick, Rhipicephalus (Boophilus) annulatus
Shawky Mohamed Aboelhadid1, Samar Mahmoud Ibrahium2, Walid Mahmoud Arafa1, Lilian Nagy Maahrous1, Abdel-Azeem Shaban Abdel-Baki3, Ahmed Anwer Wahba2
1Department of Parasitology, Animal Health Research Institute, Dokki, Egypt; 2Animal health institute, Fayoum Branch, Fayoum, Egypt; 3Zoology Department, Faculty of Science, Beni-Suef University, Egypt.
Abstract | Two entomopathogenic fungi, Verticillium lecanii and Beauveria bassiana, were tested against Rhipicephalusannulatus. Mycotal® was the source of Verticillium lecanii while Biosect® was the source of Beauveria bassiana. Five concentrations (1×107, 5×108, 2.5×109, 1×1010 and 4×1010 spore/ml) of Verticillium lecanii as well as five different concentrations (5×107, 2×108, 8×109, 3.2×1010 and 12.8x1010/ml) of Beauveria bassiana were prepared and tested against adult female tick, eggs and larvae. The mortality in adult ticks was 60.60 to 72.00% after 2 weeks of application for V. lecanii at concentration ≥ 5x108spore/ml, while B. bassiana showed no mortality at any concentrations. The treated tick revealed nutritional index significantly lower than control untreated one for both fungi. Furthermore, V. lecanii showed no effect on eggs, while, B. bassiana delayed and reduced the egg hatching. In addition, both fungi caused 100% mortality of larvae. The effective concentration was ≥ 108spore/ml for both fungi with no significant difference among the highest concentrations. Moreover, the fungal extract had no effect on adult tick. In conclusion, V. lecanii is lethal to adult tick and B. bassiana caused larvae mortality and reduced egg hatching. A prospective application of fungi of commercial source in the pasture or animal farm is possible for tick control.
Keywords | Rhipicephalusannulatus, Verticillium lecanii, Beauveria bassiana, Reproductive index, egg, larvae
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | February 01, 2018; Accepted | March 02, 2018; Published | March 25, 2018
*Correspondence | Shawky Mohamed Aboelhadid, Department of Parasitology, Animal Health Research Institute, Dokki, Egypt; Email: [email protected]
Citation | Aboelhadid SM, Ibrahium SM, Arafa WM, Maahrous LN, Abdel-Baki AAS, Wahba AA (2018). In vitro efficacy of verticillium lecanii and beauveria bassiana of commercial source against cattle tick, rhipicephalus (boophilus) annulatus. Adv. Anim. Vet. Sci. 6(3): 139-147.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.3.139.147
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Aboelhadid et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Ticks are an important threat to livestock due to either their direct effect or their role as tick-borne diseases. The ticks control is commonly by using chemical products, which has several side effects. Therefore, using biological control agents has become urgent and safe alternative to reduce and to avoid these adverse effects (Samish et al., 2004; Reis-Menini et al., 2008).
Entomopathogenic fungi were commonly used in pest control of crops and forest pests (Kaaya et al., 1996). Recently, great attention is being paid to these fungi in control of arthropods borne disease of human and animals. These fungi can infect and often kill ticks and therefore can be used in ticks control (Gindin et al., 2001). Beauveria bassiana and Lecanicillium lecanii (=Verticillium lecanii) are among the most important species infecting ixoidid ticks in nature and different strains of B. bassiana are pathogenic to different kinds of tick (Fernandes et al., 2012; Camargo et al., 2012; Ren et al., 2016). The efficacy of used the fungi depend on the tick species and population and the fungal strain (Fernandes et al., 2012; Campos et al., 2010; Sun et al., 2011; Perinotto et al., 2012). The biopesticide that contains B. bassiana (Balsamo, Vuillemin) is used to control flies on animal species (balEnce), and against crop pests’ greenhouses and food crops (Met52) (Gonzalez et al., 2016).While, Lecanicillium lecanii (Zimmermann) Gams and Zare [Verticillium lecanii (Zimmermann) Vi´egas], are well known as pathogen of arthropods with a broad host range and applied for control of whiteflies in field crops (Ravensberg et al., 1990; Osborneand Landa, 1992).The development in experiments led to release of two commercial products “Vertalec®” and “Mycotal®” based on strains specifically selected for use against aphids and whiteflies (Gardner et al., 1984; Ramakers, 1989). The efficacy of these products was approved against larvae and adult of Colorado potato beetle and Aphids (Frournier and Brodeur 2000; Kim et al., 2005; Öztürk et al., 2015).
Therefore, the aim of the present study was to assess the in vitro efficacy of two fungi at different concentrations of commercial source against eggs, larvae and adult stages of R. annulatus.
Material and methods
Ticks Collection and Preparation of Eggs and Larvae
Rhipicephalus (Boophilus) annulatus adult engorged female ticks were collected from the predilection sites of ticks; head of animal, outer and inner site of ears, neck, fore and hind limbs, abdomen and udder from naturally infested cattle from veterinary units and clinics and cow farms in Fayoum governorate in the hot seasons during the period from September 2015 to June 2017. Ticks were removed carefully from the animal by blunt and medium sized entomological forceps to pull ticks strongly from host tissue (Walker et al., 2003). The collected ticks were kept in labeled carton or plastic boxes with opening for ventilation and transported to the laboratory of Parasitology, Faculty of veterinary medicine, Beni-Suef University, Egypt. In the lab, only engorged female ticks were washed in distilled water and allowed to dry on filter paper. The collected ticks were identified according to Hoogstraal and Kaiser (1958); Arthur (1960); Fahmy (1980) and Walker et al. (2003) as Rhipicephalus (Boophilus) annulatus have anterior and short mouthpart, no festoons and medium in size (3-5mm) by using stereo binocular microscope.Then the ticks were weighted and divided into groups with 10 adult female ticks in each group. Part of these ticks was kept in BOD incubator for egg ovi-position (14-18 day). The eggs were collected into glass tubes then sealed by cotton to be used for bioassay on eggs. Another part of eggs was incubated in BOD incubator until hatching to give larvae that were used for bioassay on larvae.
The Source of Entemopathogenic Fungi in this Study
Mycotal®(as source of Verticillium lecanii preparation) is a commercial product produced by Koppert Biological Systems (Veilingweg, the Netherlands) and used for biological control in agriculture field. This product contains 1010 spores/gram entomopathogenic fungus V. lecanii. A Stock suspension of this product was prepared by adding 20 gm of the product powder in 50 ml of distilled water in a plastic tube then mixing well using vortex and wait 30 min before use (Aqueel and Leather 2013).The following concentrations were prepared from this stock;1×107, 5×108, 2.5×109, 1×1010 and 4×1010 spore/ml. These dilutions were 10ml solution diluted with distilled water.
Biosect® as Source of Beauveria Bassiana Preparations
Biosect® is a commercial product manufactured by Organic Bio-Technology (S.A.E), Egyptian Company and used for biological control in the agriculture field. Biosect® contains B.bassiana 32×106spore per mg. A stock suspension was prepared by adding 20gm of Biosect® product to 50ml distilled water then agitated by vortex to prepare the suspension. Five concentrations were prepared from this stoke; 5×107, 2×108, 8×109, 3×1010 and 12×1010spore/ml DW.
Viability of Fungi in the Used Products
Entomopathogenic fungi formulations of Mycotal® and Biosect® were prepared as mentioned above. An inoculation from each suspension was inoculated on sabourauds dextrose agar (SDA) plates, and then incubated in dark incubator at 26-28°C and 80% relative humidity for 7-10 days. Slides stained with cotton blue (Lactophenol blue stain) were prepared and examined under a microscope for viability assay (Hasan et al., 2013).
Application on Adult Engorged Females Tick
The previously prepared suspensions of V. lecanii and B. bassiana were tested on engorged female ticks of the same size. The ticks were immersed in 10ml of the fungal suspension for 2 minutes then dried and incubated in BOD for 21 days in Petri dishes (Sun et al., 2011). Five replicates each one of 10 ticks was done for each suspension. A control group was treated by immersion in 10ml DW for 2 minutes. The mortality was observed daily and the biological parameters were recorded for each group. In addition, the reproductive efficiency (RE) and other biological parameters were calculated (Bennett, 1974).
Application on the Eggs
The eggs were collected in 10 days of oviposition to be used for application of fungal suspensions. The collected eggs were subdivided into test tubes each contained 50mg. Then, 1ml of the prepared suspension was poured into each tube and kept for 3min. The tubes were turned upside down to remove any excess of the suspension with a cotton plug to absorb it. The tubes were incubated at 27 ± 1 °C and RH ≥80%. The incubation period and hatching percentage were calculated. The control group was immersed in 1ml distilled water by the same technique (Angelo et al., 2010; Camargo, et al., 2012).
Application on the Larvae
The larvae of 7-14 days age were used for bioassay. A filter paper was put in Petri dish then 1 ml of prepared fungi suspensions was added on it. The impregnated paper was allowed to dry. The treated papers were folded to form a packet and larvae were transferred by brush then sealed by bulldog clips. The treated packets were kept in controlled environment chamber at 26-28°C and 80% relative humidity for 7 days. In the control group, filter paper impregnated with 1 ml distilled water (Pirali-Kheirabadi et al., 2007).
Fungal Extract Production
The fungal suspensions of V. lecanii and B. bassiana were prepared as above. An inoculation was done on sabourauds dextrose agar (SDA) plates then it was incubated in dark incubator at 26-28 °C and 80% relative humidity for 2 weeks. The grown colonies were sub-cultured on sabourauds dextrose broth in glass tubes for another 2 weeks. After fungal growth in broth, the tubes were centrifuged at 3000 rpm for 10 min to precipitate fungal hyphae. Then, the collected supernatant was applied on adult tick and larvae by the same techniques as mentioned previously.
Statistical analysis
Data of tick biological parameters were analyzed statistically using Statistical Package for Social Science (SPSS for Windows (IBM), version 22, Chicago, USA) to determine if variables differed between treatments. In addition, ANOVA tests were applied to determine the differences between means. Results are expressed as means ± SE. Probability values of less than 0.05 (P < 0.05) were considered significant.
Results
Confirmation the fungal viability of the used products Verticillium lecanii (Mycotal®) and Beauveria Bassiana (Biosect®)were grown on the culture media that confirmed fungal viability.
Effect of V. lecanii and B. bassiana on the Adult Female of R. annulatus
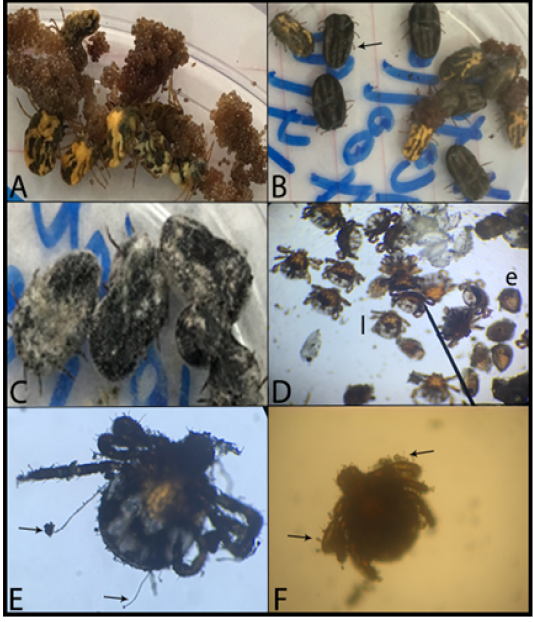
V. lecanii application at concentration of 1×107spore/ml DW on the ticks adult female revealed no any effect. While, the concentrations of ≥ 5x108 spore/ml DW processed a lethal effect on adult tick with mortality percent ranged from 60.60 to 72.00%. The fungal growth was obvious on the dead ticks after 2 weeks post application (PA)(Figure 1 B and C).In addition, these concentrations affected the biological parameters of the ticks, where NI of the treated ticks was significantly lower than control untreated one (p<0.05). Moreover, the control percent (CP) of the treated ticks at the previous concentrations was better and higher than control untreated ones. It was noticed that the concentrations of ≥5x108spore/ml DW have the same effect with no significant difference in between (p<0.05) (Table 1). In addition, the pre-oviposition, oviposition and incubation periods of the produced eggs were in the normal time as that of control untreated ticks (nearly 14 days).Meanwhile, B. bassiana application on adult ticks revealed no lethal effect at any concentrations. Furthermore, the lowest concentration of 5x107spore/ml DW has no effect on the treated ticks. While, the highest concentrations of ≥ 2x108spore/ml DW showed significant effects on the biological parameters. Where the NI of the treated ticks was significantly lower than those of the control untreated ones. Moreover, CP was higher for the treated ticks than control untreated ones (P < 0.05) with no significant difference in between these concentrations (Table 2). Additionally, we did not observe any fungal growth on adult tick. Additionally, the pre-oviposition, oviposition and incubation periods of the produced eggs were in the normal time same as of the control untreated ticks.
A. Untreated tick females deposited eggs; B. Treated ticks with arrow referringto dead tickin black color; C. Dead tickswith fungal growth; D. Treat eggs showing hatched larvae (l) and un-hatched eggs(e); E. Larvae withfungal hyphae of V. lecanii (arrow); G. Larvae with fungal hyphae of B. bassiana (arrow)
Table 1: Effect of Verticillium lecanii of commercial source at different concentrations on adult ticks Rhipicephalus annulatus at day 14 post applications
| CP | NI | RE | HP | EPI | RI | EPF | FWAT | FWBT | DF | Conc. spore/ml |
| 000 ± 000 | 72.41 ± 9.091 | 27.62 ± 2.659 | 96.00 ± 1.000 | 28.74 ± 2.507 | 0.478 ± 0.029 | 0.023 ± 0.001 | 0.047 ± 0.002 | 0.079 ± 0.004 | 00 ± 00 |
1x107 |
| 91.89 ± 0.180* | 3.58 ± 0.200* |
3.881 ± 1.111* |
92.20 ± 0.97 |
4.194 ± 1.189* |
0.010 ± 0.034* |
0.003 ± 0.001* |
0.035 ± 0.003 | 0.076± 0.003 |
6.60 ± 0.400* |
5x108 |
| 93.89 ± 0.178* | 3.578 ± 0.201* |
2.107 ± 0.061* |
91.50 ± 1.322 |
2.305 ± 0.072* |
0.072 ± 0.014* |
0.002 ± 0.000* |
0.039 ± 0.009 | 0.089 ± 0.006 |
6.80 ± 0.374* |
2.5x109 |
| 89.50 ± 1.911* | 7.104 ± 1.215* |
3.620 ± 0.659* |
92.80 ± 1.157 |
3.885 ± 0.678* |
0.089 ± 0.016* |
0.003 ± 0.001* |
0.033 ± 0.002 | 0.076 ± 0.005 |
7.000 ± 0.316* |
1x1010 |
| 88.50 ± 0.382* | 7.384 ± 0.263* |
3.966 ± 0.132* |
93.80 ± 0.583 |
4.228 ± 0.134* |
0.101 ± 0.007* |
0.005 ± 0.000* |
0.045 ± 0.001 | 0.106 ± 0.005 |
7.200 ± 0.374* |
4x1010 |
| 000 ± 000* | 62.94 ± 9.290* |
34.51 ± 3.062* |
96.50 ± 0.401 |
35.71 ± 3.104* |
1.041 ± 0.130* |
0.024 ± 0.003* |
0.027 ± 0.004 | 0.072 ± 0.009 |
00 ± 00* |
Control |
*Significant p≤ 0.05
Data expressed as (Mean ± Standard Error), conc. (spores/ml DW).
DF= Number of deaths of adult female, FWBT= Female weight before treatment, FWAT=Female weight after treatment, EPF= Egg mass per female, RI= Reproductive index, EPI= Egg production index, HP= Hatchability percentage, RE= Reproductive efficacy, NI= Nutritional index, CP= Control percent
Table 2: Effect of Beauveria bassiana of commercial source at different concentrations on adult ticks Rhipicephalus annulatus at day 14 post applications
| CP | NI | RE | HP | EPI | RI | EPF | FWAT | FWBT | DF | Conc. spore/ml | ||||||||
| 000 ± 000 | 58.31 ± 11.62 | 36.77 ± 3.812 | 93.50 ± 1.190 | 34.03 ± 2.852 | 1.096 ± 0.086 | 0.022 ± 0.002 | 0.013 ± 0.001 | 0.047 ± 0.004 | 0000 ± 0000 |
5x107 |
||||||||
| 87.51 ± 1.649* | 6.403 ± 0.785* |
4.560 ± 0.602* |
91.20 ± 0.750 |
4.990 ± 0.628* |
0.235 ± 0.037* |
0.003 ± 0.001* |
0.013 ± 0.001 | 0.059 ± 0.002 | 0000 ± 0000 |
2x108 |
||||||||
| 74.30 ± 2.989* | 13.87 ± 2.293* |
9.383 ± 1.091* |
93.50 ± 1.190 |
10.05 ± 1.172* |
0.041 ± 0.037* |
0.005 ± 0.000* |
0.013 ± 0.001 | 0.055 ± 0.006 | 0000 ± 0000 |
8x109 |
||||||||
| 75.78 ± 3.238* | 13.77 ± 2.679* |
8.840 ± 1.181* |
92.00 ± 1.224 |
9.625 ± 1.319* |
0.353 ± 0.027* |
0.005 ± 0.000* |
0.014 ± 0.001 | 0.052 ± 0.007 | 0000 ± 0000 |
3.2x1010 |
||||||||
| 84.15 ± 2.012* | 12.16 ± 3.704* |
10.52 ± 3.119* |
92.00 ± 0.707 |
11.47 ± 3.414* |
0.259 ± 0.069* |
0.008 ± 0.003* |
0.029 ± 0.002 | 0.067 ± 0.004 | 0000 ± 0000 |
12.8x1010 |
||||||||
| 000 ± 000* | 57.77 ± 7.633* |
36.46 ± 3.826* |
96.50 ± 0.422 |
37.81 ± 3.966* |
1.163 ± 0.089* |
0.020 ± 0.001* |
0.018 ± 0.001 | 0.055 ± 0.004 | 0000 ± 0000 | Control | ||||||||
*Significant at p≤0.05
Data expressed as (Mean ± Standard Error), conc. (spores/ml DW); Egg per female= 000 (Significant)
Effect of V. lecanii and B. bassiana on Eggs and Larvae
The application of V. lecanii on the egg of ticks showed no significant effect at all concentrations on either hatchability percentage or period of hatchability. As well as, B. bassiana showed no significant on eggs at the concentration of 5×107spore/ml DW where it hatched after nearly 14 days of application. While, the concentrations of ≥ 2x108spore delayed the egg hatching to 21.0 ± 0.41days post application. In addition, the hatchability percent ranged from 65.00 to 72.50% to the treated eggs at these highest concentrations. Furthermore, there was no significant difference among the effect of highest concentrations (Figure 1D) (Table 3). Regarding the treatment of larvae with V. lecanii, the lowest concentration 1×107spore/ml DW was of no effect on larval mortality and no significant difference with the control untreated larvae. The mortality effect was appeared at the concentrations of ≥ 5x108spore after 4 days post application. The mortality rate 100% was achieved at the seventh day PA (Table 4) with obvious fungal growth on the larvae (Figure 1E). Furthermore, B. bassiana effect appeared at the third day PA and100% larvae mortality was encountered at the fifth day PA (Table 4). This effect appeared at the highest concentrations of ≥ 2×108spore (Table 4).
Fungal Extracts Evaluation
The supernatants that obtained from fungal broth were
Table 3: Effect of Verticillium lecanii and Beauveria bassiana on Rhipicephalus annulatus eggs
|
V.lecanii Conc. (spore/ml) |
Hatchability percentage | Period of hatching (Days) | Comment |
|
1x107 |
98.2 ± 0.73 | 14.6 ± 0.24 | Normal hatching |
|
5x108 |
97.8 ± 0.37 | 14.8 ± 0.20 | Normal hatching |
|
2.5x109 |
98.4 ± 0.51 | 14.2 ± 0.37 | Normal hatching |
|
1x1010 |
97.8 ± 0.37 | 15.0 ± 0.00 | Normal hatching |
|
4x1010 |
98.2 ± 0.37 | 14.6 ± 0.24 | Normal hatching |
| Control | 98.8 ± 0.37 | 14.8 ± 0.20 | Normal hatching |
|
B.bassiana Conc. (spore/ml) |
Hatchability percentage | Period of hatching (Days) | Comment |
|
5x107 |
98.5 ± 0.62 |
14.7 ± 0.25 |
Normal hatching |
|
2x108 |
68.2* ± 3.11 | 21.2* ± 0.47 | Delayed hatching |
|
8x109 |
65.0* ± 3.67 | 21.2* ± 0.25 | Delayed hatching |
|
3.2x1010 |
71.0* ± 1.22 | 21.0* ± 0.41 | Delayed hatching |
|
12.8x1010 |
72.5* ± 1.50 | 21.0* ± 0.41 | Delayed hatching |
| Control | 98.2* ± 0.63 | 14.7* ± 0.25 | Normal hatching |
*Significant at p≤ 0.05
Data expressed as (Mean ± Standard Error), conc. (spores/ml DW).
Table 4: Effect of Verticillium lecanii and Beauveria bassiana on the larvae of Rhipicephalus annulatus
|
V.lecanii Conc. (spore/ml) |
% of Larval mortality at 24 hrs PA | % of Larval mortality at 48 hrs PA | % of Larval mortality at day 7 PA |
|
1x107 |
2.30 ± 0.01 | 2.70 ± 0.03 | 3.20 ± 0.01 |
|
5x108 |
1.55 ± 0.05 | 2.06 ± 0.05 | 100* ± 0.00 |
|
2.5x109 |
2.40 ± 0.02 | 2.70 ± 0.03 | 100* ± 0.00 |
|
1x1010 |
1.56 ± 0.05 | 2.46 ± 0.05 | 100* ± 0.00 |
|
4x1010 |
2.30 ± 0.05 | 3.10 ± 0.03 | 100* ± 0.00 |
| Control | 2.50 ± 0.04 | 3.20 ± 0.04 | 4.10* ± 0.00 |
|
B. bassiana Conc. (spore/ml) |
% of Larval mortality at 24 hrs PA | % of Larval mortality at 48 hrs PA | % of Larval mortality at day 7 PA |
|
5x107 |
3.40 ± 0.03 | 4.50 ± 0.03 | 5.01 ± 0.03 |
|
2x108 |
54.0* ± 1.68 | 75.7* ± 0.47 | 100* ± 0.00 |
|
8x109 |
55.2* ± 2.28 | 78.5* ± 0.95 | 100* ± 0.00 |
|
3.2x1010 |
54.0* ± 1.68 | 76.5* ± 0.64 | 100* ± 0.00 |
|
12.8x1010 |
55.5* ± 1.55 | 77.0* ± 1.08 | 100* ± 0.00 |
| Control | 3.20* ± 0.02 | 4.10* ± 0.01 |
4.70* ± 0.05 |
*Significant at p≤ 0.05
Data expressed as (Mean ± Standard Error), conc. (spores/ml DW).
applied on adult tick and showed no any efficacy with biological parameters same as these of control untreated tick.
Discussion
The bioinsecticides are commercial products containing entomopathogenic fungi and are widely used in control of insects in agriculture. These products are available and effective. Therefore, these products could be a source for fungi that can be tested against the most threatening ectoparasites of cattle in the tropical area, R. annulatus. Mycotal® is a commercial product containing Verticillium lecanii and is currently used in agriculture for control of whiteflies in crops (tomato, bean).The other product is Biosect® that is the source of Beauveria bassiana, which is commonly used against white fly in tomato.
In the present study, the in vitro effects of V. lecanii on adult R. annulatus, eggs and larvae were evaluated. The concentration of 1×107spore/ml did not show any effect on the adult ticks. While the highest concentrations ≥ 5×108spore/ml showed significant effect which appeared as death and decrease in the reproductive efficacy of ticks. After 14 days, post application (PA) 60.6-72% of treated ticks was died and fungal growth was detected on ticks. The reproductive index of the treated ticks was significantly lower than those of the control untreated group and the group of ticks treated by 1x107spore. In the same way after 7 days PA, the treated larvae died at the highest concentrations ≥ 5x108spores and fungus grow well of on the treated larvae. Meanwhile, no difference in the eggs hatching was observed between the treated eggs and the control ones at all concentrations. Therefore, V. lecanii has no effect on R. annulatus eggs. It is worthy to mention that there was a clear growth for the fungal hyphae on adult tick, which may be the main cause for death of the adult tick. The mechanism of tick infection by entomopathogenic fungi has described through germination on cuticle then penetration of it using enzymatic and physical activities of the fungus, proliferation of fungal mycelia in tick’s hemocoel and production of toxic metabolites. These led to breakdown of integument and tick death (Kirkland et al., 2004; Arruda et al., 2005; Leemon and Jonsson 2008). In the present study, the mortality rate in the treated ticks was 60.60-72.00%, which closely similar to those reported by Pirali-Kheirabdi et al. (2007) as they recorded 56.6% mortality rate in Rhipicephalus (Boophilus) annulatus ticks after application of V. lecanii at concentration of 107conidia/ml. In contrary to our results, Pirali-Kheirabdi et al. (2007) found that, the application of V. lecanii on tick eggs decrease the egg hatchability by 59.74%. Furthermore, our rate of mortality of ticks differed from those reported by Angelo et al. (2010) who applied V. lecanii on engorged females of Rhipicephalus microplus and recorded 97.6% mortality at concentration 1× 108 conidia/ml oil suspension. This difference may be due to different tick species and they used oil suspension rather than water that used in our study. In addition, Angelo et al. (2010) used L. lecanii, isolate CG 420 (laboratory strain). Furthermore, the pre-oviposition period was same as the control untreated group at all used conidial concentrations, which is similar to the results of Angelo et al. (2010) at conidia (108) aqueous suspension. In addition, the oviposition and egg incubation periods for treated engorged females were same as control in our study but it was significantly decreased for females treated in Angelo’s study. Also, the period of hatching and the percentage of hatching did not differ from the control group in both studies. Regarding the application on the larvae, both studies showed the same effects but the larvae highest mortality percent 100% was at day 7 after treatment in our study while it was at day 10 post-treatment in Angelo’s study. Furthermore, Angelo et al. (2010) found that conidia in oil suspension caused death of adult R. microplus, stopped the egg hatching and led to 100% mortality of larvae. While, our findings are somewhat differ from the findings of Gindin et al. (2001). The later found that 1×107 conidia ml−1 of L. lecanii caused reduction in the egg production of R. annulatus, Rhipicephalus sanguineus and Hyalomma excavatum with no significant mortality in larvae of R. annulatus. Nevertheless, Gindin et al. (2001) have same results like us upon application on eggs. Several authors attributed the contrast in the results to the fungal isolates, manipulation, and cultivation of these organisms in the lab (Fernandes and Bittencourt 2008; Sun et al., 2011; Perinottoet al., 2012; Ren et al., 2012; Ren et al., 2016).Similarly, the fluctuation in our results in comparison with the previous studies might be due to the nature of the source of fungi that it is of commercial product lyophilized powder and using aqueous suspension as recommended by the manufacturer.
Beauveria bassiana is one of the important entomopathogenic fungi used in biological control in agriculture field. In the present study B. bassiana showed no lethal effect on adult female of R. annulatus at any concentration. However, a significant (p <0.05) reduction in biological parameters and significant reduction in the eggs hatchability percentage with prolonged hatchability period when compared with the control untreated group (21.2 ± 0.47 day) were noticed at concentrations ≥2×108spore/ml. Furthermore, treatment of larvae caused significant mortality at concentrations ≥2x108spore and the mortality percentage reached to 100% after 5 days PA. In contrast to our study, Kaaya et al. (1996) recorded 30% and 37% mortality of Rhipicephalus appendiculatus and Amblyomma variegatum respectively after application of B. bassiana. In addition, Kaaya and Hassan (2000) reported 80-90% mortality in adults Rhipicephalus appendiculatus, Amblyomma variegatum and Boophilus decoloratus using aqueous and oil-based formulations of Beauveria bassiana. In the same way, Ren et al. (2011) recorded 100% mortality rate at 108 conidia ml-1inHaemaphysalis qinghaiensis ticks in china. In addition, Ren et al. (2012) reported that three B. bassiana isolates caused up to 100% mortality for the ticks at concentrations of 108 and 109 conidia mL–1. Moreover, Cafarchia et al. (2015) estimated high significant mortality rate in the adult of Rhipicephalus sanguineus sensu lato. Similarly, Murigu et al. (2016) tested B.bassiana against amitraz-resistant and amitraz-susceptible strains of Rhipicephalus decoloratus and they found a significant reduction in the number of ticks and larval mortality (10-100% and 12.1-100%) in both respects. Consequently, this discrepancy with our findings may be due to the virulence of the fungus strain used in the present study, which is isolated from Egyptian soil as in the label of manufacturing. In addition, Fernandes et al. (2011) tested the virulence of sixty Beauveria-like isolates on R. microplus larva and verified that larvae from different origins had different susceptibilities to the fungal isolates. Moreover, Perinotto (2010) reported variation in susceptibility of R. microplus larval stages to B. bassiana and M. anisopliae when the ticks were collected from different locations. Additionally, the difference in susceptibility of R. microplus to entomopathogenic fungi related to genetic and physiological characteristics of the tick strains and species (Fernandes et al., 2012). Moreover, Polar et al., (2005) reported that ticks might be physically and structurally tolerant to infection by entomopathogenic fungi. Besides, the previous exposure to entomopathogenic fungi cannot discard in nature where these fungi present in the soil but there is no record of epizootic naturally in ticks (Prinitto et al., 2012).
Herein, the mortality of larvae was 100% at 5 days PA. It was known that tick larvae were the most susceptible than other stages (Camargo et al., 2012).The present result is similar to those reported by Cafarchia et al., (2015) but they encountered 100% mortality of larvae at 15 days PA. On other hand, several studies reported lower mortalities rates than the rate of present study (Kaaya and Hassan 2000; Reis et al., 2005; 2012; Campos et al., 2010). We think that the technique of application, larval age and tick species may be the causes of this variation.
The results of the application on eggs led to reduction in the percentage of hatching and delaying in the hatching. These results are similar to that obtained by Kaaya and Hassan (2000) and Cafarchia et al. (2015). In the present study, the application of B. bassiana of commercial origin was greatly effective on larvae. This is in agreement with González et al., (2016) who found that the application of B. bassiana commercial strain (Balsamo, Vuillemin) on Oryctolagus cuniculus (Lagomorpha: Leporidae) wild rabbit burrows under field conditions in aqueous solutions of the product reduced the infestation by 63.28 =78.63% % on day +30 and +60, respectively.
In the present study, the effective concentrations of fungi of the significant effect were ≥ 2×108spore/mlwhile the concentration of 1×107spore/ml was not effective. This in agreement with Polar et al. (2005) who found that high concentration of fungal conidia is necessary to cause its lethal effect on tick. Moreover, Prette et al. (2005) found that the application of 109 conidia/ml of B. bassiana isolates was significantly reduced the hatching of larvae compared to the concentrations of 107 conidia mL-1.In addition, ticks seem to be naturally more tolerant to fungal infection than many other arthropods, therefore, high conidial concentrations are needed to achieve the lethal effect (Polar et al., 2005b). Moreover, Maniania et al. (2007) reported that tick control needs very high concentrations in comparison with the fungal concentration used to control important agricultural insect pests. Similarly, Ren et al. (2012) reported that concentrations of 108 and 109 conidia was the effectiveness of B. bassiana, while the concentration 107spores mL–1 reduced only the reproductive efficacy. However, in the present study, the concentrations of 109 and 1010spore/ml showed no significant difference with the result of concentration of 108spore. This may be due to fungi occupy all body of the tick at the concentration of 108 spore and any increase in conidia concentration do not find a place to act or grow on the tick.
Regarding, the fungal extracts application on adult tick had no effect. This may support that the action of fungi on tick mainly due to its growth and not to its metabolites. This in agreement with Moon et al. (2008) who found that M. acridum has very low destruxin production even though this isolate is very effective against grasshopper/locust. Moreover, Golo et al. (2011) found that different concentrations of destruxin A did not cause any effect on engorged female of R. microplus. Additionally, Fernandes et al. (2012) concluded that the fungal production of toxic metabolites might not be crucial for pathogenicity to arthropods, including ticks.
In conclusion, V. lecanii had a lethal effect on adult R. annulatus and larvae but had no effect on eggs. This finding is interest and need field application. While B. bassiana had no lethal effect on adult R. annulatus but reduced its reproductive efficacy and its eggs hatchability and caused mortality for the larvae. Concurrently, a recommendation for using these fungi in its commercial products and as aqueous suspensions on tick-infested pasture may be of significant effect. This issued by Ojeda-Chi et al. (2010) who applied this strategy previously. Therefore, regular application of the concentration 108conidia/ml as a spray in the pasture and animal farms may play an effective role in the control of tick larvae and eggs in the pasture.
Conflict of interest
There is no conflict of interest.
AcknowledgmentS
We appreciated grateful thank for Dr.Walid H. Hassan for helping in fungal culture and identification.
Authors Contribution
Samar M Ibrahium and Lilian N Maahrous are responsible for practical work as applications and statistical study with help of Walid M Arfa in collecting ticks sample and identification, also Ahmed A Wahba is responsible for searching and bringing the biological commercial product, while Shawky M aboelhadid and Abdel-Azeem S Abdel-Baki are helping in writing and editing the research article.
References
Rhipicephalus evertsi evertsi ticks. Exp. Appl. Acarol. 46: 149-156. https://doi.org/10.1007/s10493-008-9186-2