Advances in Animal and Veterinary Sciences
Research Article
Histopathological Effects of using Grape Seed Extract Alone or in Combination with Ofloxacin against Pasteurella Multocida in Rabbits
Sawsan M.A. El-Sheikh1, Azza A.A. Galal2 , Fatma M. Youssef3, Amina A. Dessouki4, Haidi. I. Mohamed1*
1Department of Pharmacology, Animal Health Research Institute, Ismailia, Egypt; 2Department of Pathology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt; 3Department of Clinical Pathology, Animal Health Research Institute, Ismailia, Egypt; 4 Department of Pathology, Faculty of Veterinary Medicine, Suez canal university, Ismailia, Egypt.
Abstract | In trial to investigate the effect of experimentally Pasteurella multocida , ofloxacin, Grape seed extract and both together onhistopathological changes in rabbits, Fifty weaned rabbits were divided into five groups; control negative (I), control positive (II), ofloxacin treated (III), GSE treated (IV) and combined treated groups (V). The result of histopathological changes of Pasteurella infected group showed severe peribronchitis and congested blood vessels as well as severe lymphocytic infiltrations and compensatory emphysema in lung. Liver revealed massive lymphocytic infiltration along with fibrosis around the hepatic area. Diffuse degeneration of hepatocytes and focal necrosis were also observed. Kidneys showed degeneration of renal tubules and focal interstitial aggregations with lymphocytes. The animals of group III (O) and group IV (G), showed mild to moderate lesions. The group V (O+G) showed pronounced improvement in tissues. It could be concluded that using both ofloxacin and GSE had a good effectiveness in treatment of Pasteurellosis in rabbits.
Keywords | asteurella multocida, Rabbit, Ofloxacin, GSE, Histopathological changes
Received | January 09, 2020; Accepted | March 31, 2020; Published | April 10, 2020
*Correspondence | Haidi. I. Mohamed, Department of Pharmacology, Animal Health Research Institute, Ismailia, Egypt; Email: [email protected], [email protected]
Citation | El-Sheikh SMA, Galal AAA, Youssef FM, Dessouki AA, Mohamed HI (2020). Histopathological effects of using grape seed extract alone or in combination with ofloxacin against pasteurella multocida in rabbits. Adv. Anim. Vet. Sci. 8(5): 463-471.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.5.463.471
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 El-Sheikh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Rabbits are viewed as one of the essential domesticated animals that give high quality protein sustenance. One of the most important health problems in rabbit is pasteurellosis, which is considered as a common bacterial disease caused by Pasteurella multocida (P. multocida) and has been reported as a constant serious and highly contagious disease of domestic rabbits (Nassar et al., 2013).
Rabbit pasteurellosis causes symptoms that range from fatal septicemia, severe pleuritis, and pneumonia to less severe sequelae such as multiple abscesses, chronic rhinitis, and otitis media. It mostly affects rabbits at 4–8 weeks of age causing manifestations going from lethal septicemia, serious pleuritis, and pneumonia to less extreme sequelae, for example, various abscesses, perpetual rhinitis, and otitis media. The result of any type of the ailment is extreme financial misfortunes. Rabbits older than 8months to 1 year of age showed lower incidence (Mohamed et al., 2019).
Pasteurellosis exhibited 3 forms in rabbits. The first one is snuffles or nasal catarrhal inflammation which is characterized by acute, subacute, and chronic inflammation of the air passages and lungs. This form of the disease often ends with death and the cured animals became carriers. The second form is characterized by abscess formation at any part of the body and the case is terminated with septicemia. The last form is characterized by genital infection, which manifests as acute and subacute inflammation of uterus and testicles. Also, rhinitis is the most common clinical manifestation in rabbit pasteurellosis.
The systemic fluoroquinolones (FQs) have been used for >30 years for a variety of infectious conditions, and have been among the most widely prescribed antibiotics globally (Richards et al., 2019).
Fluoroquinolones, second generation of quinolones, are amphoteric molecules with broad tissue penetration, large volume of distribution, almost full systemic bioavailability after parenteral pathways, low percentage of plasma protein binding, and low toxicity. As a result, they were regarded to be efficient drugs for the therapy of a number of bacterial diseases (Abo-el-Sooud and Goudah, 2010).
Indeed, it is recommended that these substances be used carefully in animals approved for human consumption. Their inappropriate and uncontrolled use carries potential microbial resistance hazards that can affect not only the production system, but human health and the environment as well (Quesada et al., 2013). In view of these facts, traditional medicine can offer a wealth of interesting ways to fight drug resistance (Narayanan et al., 2011; Potroz and Cho, 2015).
Herbs exhibit a variety of biological activities and can be used efficiently to manage diseases (Gupta and Birdi, 2017).
Grape (Vitisvinifera L.) is considered to be one of the major fruit crops in the world based on hectares cultivated and economic value (Torregrosa et al., 2015). Grape seeds, a by-product of the juice and wine industry, are a rich source of polyphenols (Lin et al., 2014). Grape seed extract (GSE) contains plant flavonoids such as proanthocyanidins and procyanidins which are potent antioxidants and exert many health-promoting effects. The antimicrobial effects of GSE are due to high levels of potent antioxidant polyphenols. Antibacterial activity was bactericidal as shown by a disruption of the bacterial cell wall) (Al-Habib et al., 2010). GSE can inhibit the growth of a broad spectrum of Gram-negative and Gram-positive bacteria depended on its concentrations, phenolic content, and tested bacterial species. It has also been shown to have inhibitory effects against several clinically important viruses and fungi. GSE may be a promising source for new generations of antimicrobial agents in the food industry and clinical setting (Memar et al., 2019). Therefore, this study was designed to evaluate the potential role of GSE in treating of P. multocida infection in rabbits, increasing the efficacy of ofloxacin treatment, and ameliorating its side effects.
Therefore, the present work was adopted to evaluate the protective effect of Ofloxacin, GSE when administrated alone or in combination against experimental challenge of rabbits with P. multocida strain. The study was based on histopathological investigation.
Materials and Methods
Ofloxacin
Ofloxacin antibiotic powder was obtained from Sigma Aldrich Co. (Steinhein, Germany). Infected rabbits were orally administered with ofloxacin 25 mg/ kg /twice daily (Öztürk et al., 2000).
Plant material and extraction
The grape seeds of were purchased from local market, Kasassen, Ismailia, Egypt. The selected seeds were botanically identified and authenticated in Botany Department, Faculty of Science, Suez Canal University. The grapeseeds were dried in hot air oven at 40ºC till complete dryness and pulverized to fine powder in a mechanical grinder. The powder was macerated in 70 % ethanol (25% w/v) for 3 days in dark place (25 - 30°C) and was stirred 3 times a day (Badavi et al., 2013). The extracts were then filtered through Whatman No. 1 filter paper, and the residue was repeatedly extracted with the same solvent until it was colorless. The liquid extract was collected in a dark brown bottle. The extracts were concentrated under reduced pressure in a rotary evaporator (temperature 40 °C) till complete dryness. The dried extract was collected, weighed and stored at 4°C until further use. Infected rabbits were orally administered with GSE 250 mg/ kg once daily (Benzer et al., 2012).
Pasteurella multocida strain
Pasteurella multocida strain was obtained from Animal Health Research Institute (AHRI), El Dokki Giza, Egypt. Colonies were suspended in sterile saline and the density was adjusted with a final concentration of 1X 107 colony forming unit (CFU)/mL. After one week of acclimatization, the rabbits (5 weeks of age) were randomly assigned into five equal groups (each of 10 rabbits). On 1st day of the experiment, group I (control group) intranasally administered 0.1 ml sterilized saline. In all other rabbits, snuffles were induced by intranasal injection of 0.1 ml of P. multocida (1X 107 CFU) (Jarvinen et al., 1998).
Experimental rabbits and management
A total of 50 recently weaned male New Zealand rabbits, one month age, weighing about 600±20 g were obtained from a rabbit farm in Kasaseen, Ismailia, Egypt. They were kept for one week before commencement of the experiment. They were housed in double flat galvanized wire, batteries (40×50×40cm) and were kept under the same management, hygiene and environmental conditions at the Faculty of Veterinary Medicine farm, Suez Canal University, Ismailia, Egypt. The rabbits were fed on commercial ration supplied from El-Baraka Company, Kasaseen; Ismailia. The Rabbits were checked three times daily (at 6 am, 2 pm and 10 pm) for feed, water and mortality. All experimental work was done under biosafety circumstances that fulfill with the local animal welfare rules on experiments with rabbits.
Induction of snuffle and experimental design
After one week of acclimatization, the rabbits (5 weeks of age) were randomly assigned into five equal groups (each of 10 rabbits). On 1st day of the experiment, group I (control group) intranasally administered 0.1 ml sterilized saline. In all other rabbits, snuffles were induced by intranasal injection of 0.1 ml of P. multocida (1X 107 CFU) (Jarvinen et al., 1998).
Twenty four hour later (after appearance of clinical signs), various drug regimens were initiated that continued for five consecutive days. Experimentally infected rabbits were allocated into group II (infected–non treated), infected group were orally administered with 0.5% DMSO solution; group III (infected-ofloxacin-treated), infected rabbits were orally administered with ofloxacin 25 mg/ kg /twice daily (Öztürk et al. 2000); group IV (infected- GSE-treated), infected rabbits were orally administered with GSE 250 mg/ kg once daily (Benzer et al., 2012);group V (infected-co-treated), infected rabbits were simultaneously treated with half dose of ofloxacin and GSE. The final volume of each treatment was adjusted to be 2 ml and administered using plastic syringe connected to rubber stomach tube. The rabbits in all groups were carefully observed throughout the study.
Histopathoogical analysis
Specimens from the liver, kidneys, spleen, lungs, brain and heart from normal and all treated groups were collected at the end of 7th week post-treatment, fixed in 10 % neutral buffered formalin for 48 hours, then washed overnight under running water. The washed specimens were dehydrated by using up graded concentrations of ethyl alcohol starting with 75% and ending with absolute alcohol.Cleaned in xylene two times, each for 2 h. After that, the specimens were placed in crucible containing soft paraffin and kept in an oven at 60o C for 12 h. Paraffin sections of 5 microns thickness were prepared and stained with H and E stains for histopathological examination (Levison, 1997).
Results
Clinical signs
On the second day of the experiment, group II rabbits (infected-non-treated) started to exhibit inconsistent clinical signs. These signs were mostly sneezing, nasal discharges, rough fur, labored breathing and in few cases, there were subcutaneous abscesses and also diarrhea. However, rabbits treated with ofloxacin (group III) and GSE (group IV) showed less severe clinical signs than did group II. Rabbits in infected co-treated were nearly healthy with much less severe clinical signs.
Postmortem changes
Trachea showed hyperemic mucosa with petechial to ecchymotic hemorrhages. Rabbits that died between 48 to 108 h PI showed the most severe and prominent lesions in lungs and trachea. The lesions were characterized by diffused necrosuppurative fibrinous pneumonia involving different lobes of lung with highest severity in cranial lobes. There was deposition of thick fibropurulent exudate involving the entire lobes of lung. Three rabbits showed fibrinous pneumonia characterized by moderate to severe pleural thickening, multifocal to diffuse hemorrhage. Grossly, tracheal mucosa was markedly reddened and showed multifocal white necrotic patches. Minimal amount of mucus and/or pus admixed with necrotic debris was present overlying mucosa. Pharynx and larynx mucosa was severely reddened due to congestion and hemorrhages.
Concerning Thoracic cavity, it contained serosanguinous exudates. Pericardium and other thoracic organs of all affected rabbits exhibited variable degree of fibrin deposition admixed with pus. Mediastinal lymph nodes were moderately enlarged and dark in color in all affected rabbits.
At the time of necropsy, spleen did not show any pathological changes.
Grossly, liver of all infected rabbits were diffusely congested. Grossly, meninges of infected animals were thickened and congested. The challenged animals in this study developed septicemia as evidenced by the presence and re-isolation of the challenge organism from a variety of tissues and emboli seen in capillaries of brain. Concerning kidney, severe congestion in the two lobes were found.
Histopathological changes
Lung of normal control rabbits revealed a normal bronchial and bronchiolar structure with normal alveolar and peri-alveolar capillaries. On the other hand, the lung of Pasteurella infected group showed severe peribronchitis and congested blood vessels. On the other hand, there was diffused thickening of the interstitial tissue with dilated capillaries, hemorrhages and severe lymphocytic infiltrations. Alveolar collapse, tissue destruction and compensatory emphysema were also seen. The animals of group III (O) showed mild to moderate lesions as mild congestion and leucocytic infiltration of preialveolar capillaries with mild emphysema of alveoli and mild focal necrotic lesions. Group IV had moderate thickening of perialveolar septa with lymphocytic infiltrations and congestion of perialveolar. The group V, had mild edema between alveoli with scanty inflammatory cells and no necrosis or tissue destruction as shown in Figure 1.
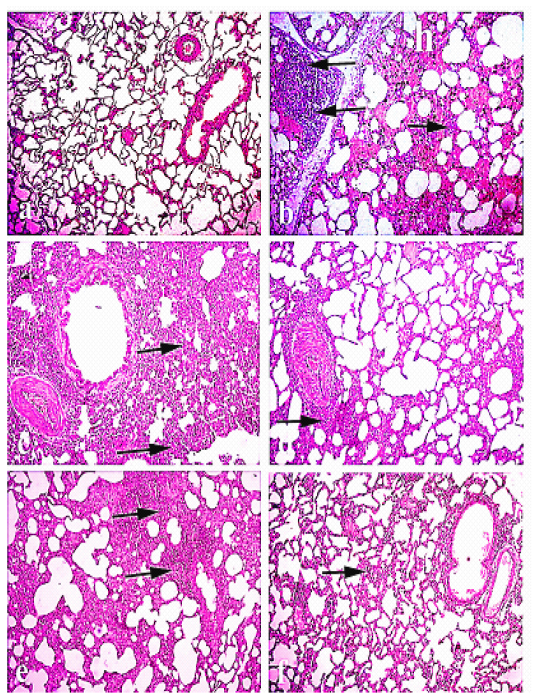
Figure 1: Lung of rabbit: (a) Lung of normal control animals. (b)and (C) lung of pasteurella infected group showing interstitial inflammatory reaction and hemorrhage, and (d) lung treated with Ofloxacin., (e)lung treated with Grape seed extract . (f ) lung treated with( O+ G). (arrows) refer to thickening of interstitial tissue with lymphocytic infiltration. Hematoxylin and eosin stain; magnification, 100×.
Liver of normal control group showed normal hepatic lobules with normal centrally located central veins. Hepatic cords were arranged in radiating manner around the central veins and had normal hexagonal hepatic cells with abundant eosinophilic cytoplasm. Livers infected with pasteurella revealed severe congestion of hepatic blood vessels, hyperplasia of bile ducts, and massive lymphocytic infiltration along with fibrosis around the hepatic area. Diffuse degeneration of hepatocytes and focal necrosis were also observed. Group received Ofloxacin showed diffuse vacuolar degeneration and mild congestion of central veins. The group received grape seed extract had focal areas of degeneration with mild congestion of blood vessels and mild fibrosis. The group received (O+G) showed a fairly normal hepatic cords with focal hepatocytes with mild vacuolar degeneration as shown in Figure 2.
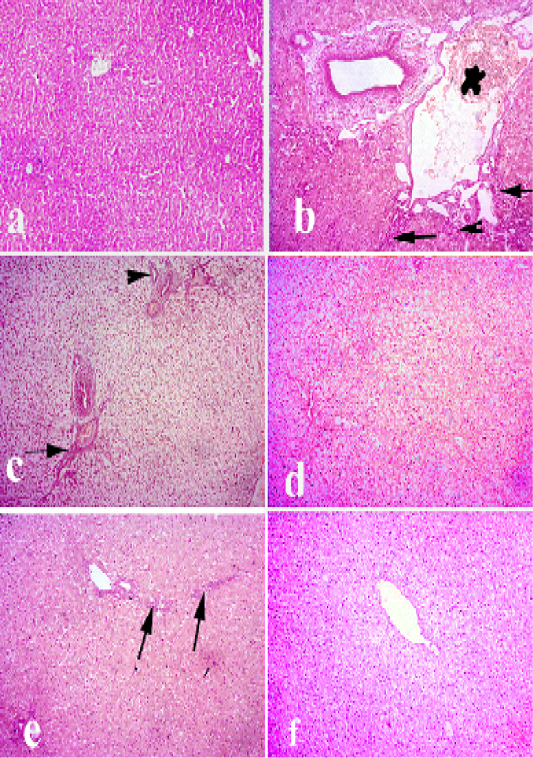
Figure 2: Liver of rabbit. (a) liver of control animals. (b and c) liver of pasteurella infected group showing degeneration. necrosis of hepatocytes and severe congestion of blood vessels, hyperplasia of bile duct and fibrosis. (d) livertreated with (Ofloxacin) showing diffuse vacuolar degeneration. (e) livertreated with grape seed extract showing mild vacuolar degeneration of hepatocytes along with fibrosis. (f) liver treated with (O+G) showing mild focal pericentral vacuolar degeneration. (arrows) refer to fibrosis and lymphocytic infiltrations, (arrow heads) refer to hyperplasia of bile ducts. Hematoxylin and eosin stain; magnification, 100×.
Kidney of control animals showed normal renaltubules with normal glomeruli. Kidney of Pasteurella infected group showed degeneration of renal tubules, proliferation of some glomeruli and focal interstitial aggregations with lymphocytes. Kidney treated with Ofloxacin showed mild vacuolar degeneration of some renal tubules. Kidney treated with (grape seed extract) showed mild vacuolar degeneration of some renal tubules along with mild lymphocytic infiltrations between the renal tubules. Kidney treated with (O+G) showed pronounced improvement of renal tissue as shown in Figure 3.
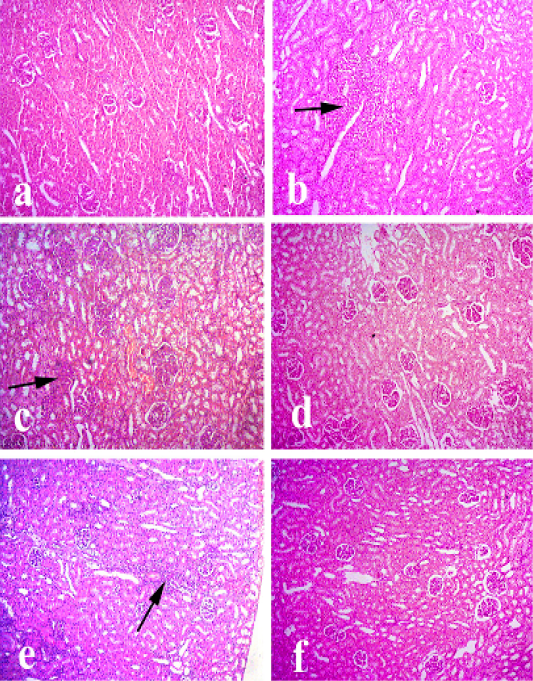
Figure 3: Kidney of rabbit. (a) Kidney of control animals. (b and c) Kidney of pasteurella infected group showing degeneration of renal tubules, proliferation of some glomeruli and focal interstitial aggregations with lymphocytes. (d) Kidney treated with (Ofloxacin) showing mild vacuolar degeneration of some renal tubules. (e) Kidney treated with grape seed extract showing mild vacuolar degeneration of some renal tubules along with mild lymphocytic infiltrations between the renal tubules. (f) Kidney treated with (O+G) showing pronounced improvement of renal tissue. (arrows) refer to lymphocytic infiltrations. Hematoxylin and eosin stain; magnification, 100×.
Heart of normal control animals showed normal heart muscles. While heart of Pasteurella infected group showed severe congestion of blood vessels and interstitial inflammatory reaction, degeneration and intermuscular edema. On the other hand, heart treated with ofloxacin showed mild congestion and mild degeneration and intermuscular edema. Heart treated with grape seed extract showed mild congestion and mild degeneration and intermuscular edema. Heart treated with (O+G) showed fairly normal histological pictureas shown in Figure 4.
Brain of normal control animals showing normal histological picture. While, brain of Pasteurella infected group showed congestion of blood vessels, lymphocytic infiltrations and degeneration of neurons. Brain treated with ofloxacin showed focal degeneration of some neurons. Brain treated with grape seed extract showing mild congestion and focal degeneration. Brain treated with (O+G) showing fairly normal pictureas shown in Figure 4.
Spleen of normal control animals showed normal white and red pulps. Spleen of Pasteurella infected group showing severe congestion and focal depletion of white pulp. Spleen treated with ofloxacin showing mild congestion of sinusoidal blood vessels. Spleen treated with grape seed extract showing mild depletion of white pulp. Spleen treated with (O+G) showing discrete depletion of some lymphocytesas shown in Figure 6.
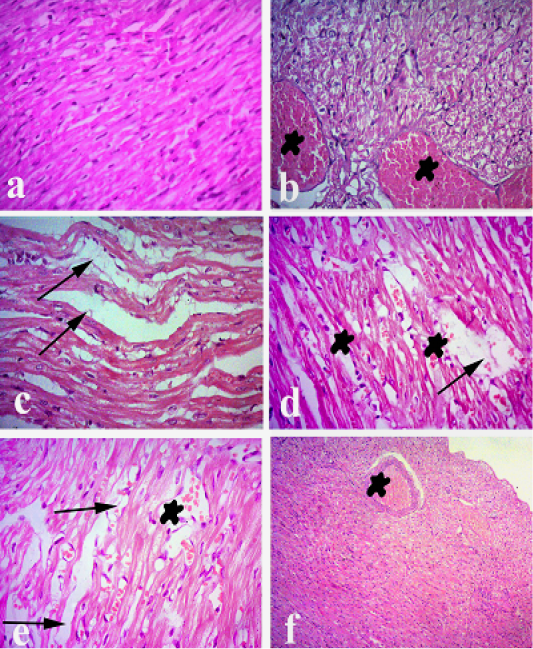
Figure 4: Heart of rabbit. (a) Heart of normal control animals showing normal heart muscels. (b and c) Heart of pasteurellainfeceted group showing severe congestion of blood vessels and interstitial inflammatory reaction, degeneration andintermuscular edema. (d) Heart treated withofloxacin showing mild congestion and mild degeneration and intermuscular edema. (e) Heart treated with grape seed extract showing mild congestion and mild degeneration and intermuscular edema. (f) Heart treated with (O+G) showing fairly normal histological picture. (arrows) refer to degeneration and intermuscular edema, (stars) refer to congestion. Hematoxylin and eosin stain; magnification, 100×.
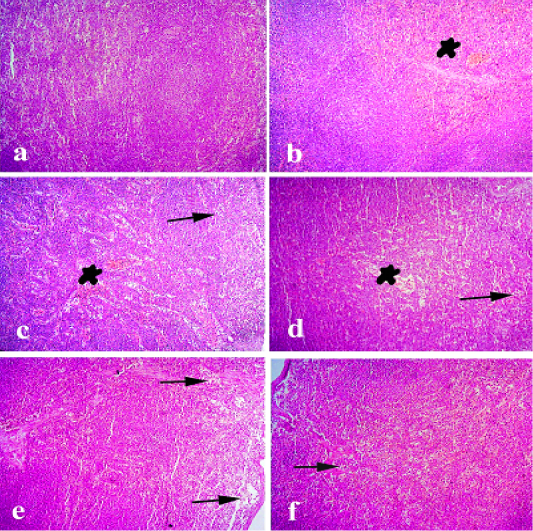
Figure 5: Spleen of rabbit. (a) Spleen of normal control animals showing normal white and red pulps. (b and c) Spleen of pasteurella infected group showing severe congestion and focal depletion of white pulp. (d) Spleen treated with ofloxacin showing mild congestion of sinusoidal blood vessels. (e) Spleen treated with grape seed extract showing mild depletion of white pulp. (f) Spleen treated with(O+G) showing discrete depletion of some lymphocytes. (arrows) refer todepletion of white pulp. (stars) refer to congestion of blood vessels. Hematoxylin and eosin stain; magnification, 100×.
Discussion
The disease caused by Pasteurella multocida is an extremely common and difficult issue of rabbits utilized for (biomedical research. P. multocida causes suppurative rhinitis snuffles), otitis media, enzootic pneumonia, conjunctivitis, pyometra, orchitis, subcutaneous abscesses, and septicemia in rabbits (Flatt, 1974).
Different methods have been endeavored to control and eliminate pasteurellosis. One of the additionally encouraging methodologies is the improvement of treatment techniques by the combination between chemotherapy and herbal therapy.
Our results were based on the histopathological findings particularly in different organs of infected, non infected and treated groups.
Concerning lung of group I a normal bronchial and bronchiolar structure with normal alveolar and peri-alveolar capillaries was shown. On the other hand, lung of group II revealed severe peribronchitis and congestion of the blood vessels. Diffused thickening of the interstitial tissue with dilated capillaries, hemorrhages and severe lymphocytic infiltrations. Alveolar collapse, tissue destruction and compensatory emphysema. Our results were in agreement with (Mohamed et al., 2019) who proved that lung of Pasteurella infected rabbit revealed extensive hemorrhage with presence of large numbers of extravasated RBCs in the alveolar lumens. (Alam et al., 2018) observed that the lung tissue of normal control rabbits exhibited normal bronchial and bronchiolar structures with normal alveolar and perialveolar capillaries. On the other hand, lung tissue
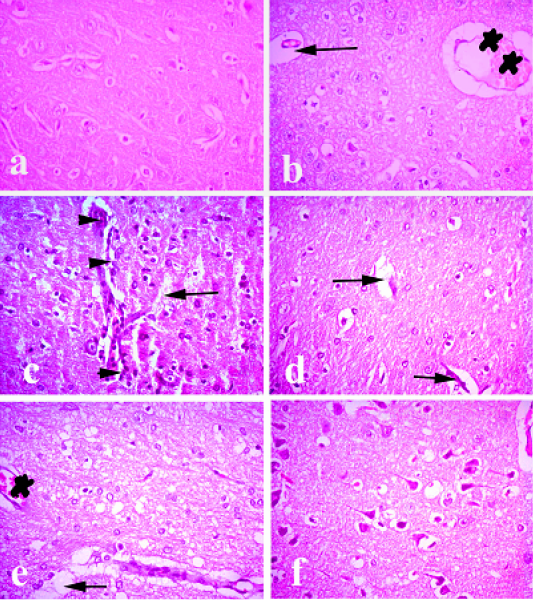
Figure 6: Brain of rabbit. (a) Brain of normal control animals showing normal histological picture. (b and c) Brain of pasteurella infected group showing congestion of blood vessels, lymphocytic infiltrations and degeneration of neurons. (d) Brain treated with ofloxacin showing focal degeneration of some neurons. (e) Brain treated with grape seed extract showing mild congestion and focal degeneration. (f) Brain treated with (O+G) showing fairly normal picture. (arrows) refer degenerated neurons, (arrow heads) refer to lymphocytic infiltration. (stars) refer to congestion. Hematoxylin and eosin stain; magnification, X 100.
sections from Pasteurella-infected rabbits showed severe peribronchitis, congested blood vessels, vascular thrombi, and vasculitis. Diffuse interstitial inflammatory reaction, alveolar collapse, tissue destruction, and compensatory emphysema. Our results were in parallel with (Eid et al., 2019) who proved that the emergency slaughtered and freshly dead duck birds showed emphysema, hemorrhages in pulmonary tissue and marked thickening of the interalveolar septa (interstitial pneumonia) characterized by hyperplasia of their lining epithelium. Infiltration with inflammatory cells as well as serofibrinous pneumonia was evident. In other cases the lung showed also severe congestion and mononuclear cells infiltrations between the alveoli and around the blood vessels.
Lung of group III showed mild to moderate lesions as mild congestion and leucocytic infiltration of preialveolar capillaries with mild emphysema of alveoli and mild focal necrotic lesions. Our results were in agreement with (Alam et al., 2018) who mentioned that rabbits that received norfloxacin after infection had mild thickening of the interalveolar septa with inflammatory cells and congestion.
Concerning group IV, moderate thickening of perialveolar septa with lymphocytic infiltrations and congestion of perialveolar was observed. In a similar study of using allicin as a herbal treatment of pasteurellosis proved by (Alam et al., 2018), the lung showedmild to moderate lesions as mild congestion and leukocytic infiltration of interalveolar capillaries with mild emphysema of alveoli and focal necrotic lesions.
Our study revealed that lung of combined treated group (group V) showed mild edema between alveoli with scanty inflammatory cells and no necrosis or tissue destruction.
Liver of Group I showed normal hepatic lobules with normal centrally located central veins. Hepatic cords were arranged in radiating manner around the central veins and had normal hexagonal hepatic cells with abundant eosinophilic cytoplasm. (Mohamed et al., 2019) proved that liver of Pasteurella infected rabbit showed peliosis and hepatitis (multiple, randomly distributed, blood-filled cavities throughout the liver. In our study, liver of group II revealed severe congestion of hepatic blood vessels, hyperplasia of bile ducts, and massive lymphocytic infiltration along with fibrosis around the hepatic area. Diffuse degeneration of hepatocytes and focal necrosis. (Eid et al., 2019) reported that the liver sections showed moderate to severe vacuolar degeneration of hepatocytes with nuclear pyknosis. Massive fat change was also observed. Necrosis, mononuclear cells infiltrations around the blood vessels and perivascular edema was also detected. chickens. meanwhile liver of ofloxacin received group (group III) showed diffuse vacuolar degeneration and mild congestion of central veins. Our result was in parallel to (Alam et al., 2018) who observed in his study that rabbits received norfloxacin after infection had focal areas of degeneration with mild congestion of blood vessels.
The group received grape seed extract had focal areas of degeneration with mild congestion of blood vessels and mild fibrosis. The group received the combined treatment showed a fairly normal hepatic cords with focal hepatocytes with mild vacuolar degeneration. (Hassan and Al-Rawi, 2013) had observed that the liver section of the animals in the group treated with GSE showed appearance of improvement of the central vein structure and the portal region.
Concerning kidney of normal rabbits (group I), normal renal tubules with normal glomeruli was observed. (Hassan and Al-Rawi, 2013) proved the same result in the control group. Group II showed degeneration of renal tubules, proliferation of some glomeruli and focal interstitial aggregations with lymphocytes. vascular congestion and severe hydropic degeneration in the epithelial lining of the tubular epithelium of kidney were recorded (Mohamed et al., 2019).
The kidney showed edema, severe congestion along with tubular nephrosis and marked coagulative necrosis (El-Demerdash et al., 2015). Kidney treated with (Ofloxacin) showed mild vacuolar degeneration of some renal tubules. Kidney treated with (grape seed extract) showed mild vacuolar degeneration of some renal tubules along with mild lymphocytic infiltrations between the renal tubules. (Hassan and Al-Rawi, 2013) proved that the kidney treated with GSE showed the normal structure of the kidney as control Kidney treated with (O+G) showed pronounced improvement of renal tissue.
Heart of control group showed normal heart muscles while heart of group II showed severe congestion of blood vessels and interstitial inflammatory reaction, degeneration and intermuscular edema. (Mohamed et al., 2019) recorded extensive hemorrhages, intermuscular edema, with vacuolation of the cardiomyocytes in heart of Pasteurella infected group. (Wada et al., 2010) reported mild to moderate congestion of blood vessels and hemorrhage in the intermycium of the heart in cases of fowl cholera. The heart showed edema among the degenerated and necroses of cardiac muscle bundles in few cases, the heart showed myocardial edema and serofibrinous to organized pericarditis, myocardial degeneration with swollen granular myocytes at 36 hours after experimental infection of broiler birds by Pasteurella. On the other hand, heart treated with ofloxacin showed mild congestion and mild degeneration and intermuscular edema. Heart treated with grape seed extract showed mild congestion and mild degeneration and intermuscular edema. Heart treated with (O+G) showed fairly normal histological picture.
Brain of control group showed normal histological picture. While, brain of Pasteurella infected group showed congestion of blood vessels, lymphocytic infiltrations and degeneration of neurons. Brain showed widespread areas of congestion, neuronal degeneration and focal gliosis (Fatma, 1999). Brain treated with ofloxacin showing focal degeneration of some neurons. Brain treated with grape seed extract showing mild congestion and focal degeneration. Brain treated with (O+G) showing fairly normal picture.
Spleen of control group showed normal white and red pulps. On the other hand, spleen of infected non treated group showed severe congestion and focal depletion of white pulp. our study was in agreement of (Mohamed et al., 2019) who observed severe lymphoid depletion of the lymphoid follicle associated with hemosiderosis. Spleen showed necrosis, edema and lymphoid depletion of lymphoid follicles of the white pulp (El-Demerdash et al., 2015). Spleen treated with ofloxacin showing mild congestion of sinusoidal blood vessels. Spleen treated with grape seed extract showing mild depletion of white pulp. Spleen treated with(O+G) showing discrete depletion of some lymphocytes. (Palócz et al., 2014) revealed thatautopsy revealed congested heart accompanied with enlarged and congested blood vessels, necrotic foci in the liver, brown peritoneum, congested friable kidneys, and dark brown spleen. Trachea, lungs, and heart were congested, hyperemic and filled with blood. The lungs showed peribronchitis, severely congested vessels with vasculitis, marked alveolar collapse, diffused interstitial inflammatory reaction and intra-alveolar tissue destruction. Positive control and β-glucan animals showed similar pathology. The enrofloxacin treated group had milder symptoms; interstitial pneumonia and Pasteurella septicemia occurred but necrotic lesions and macroscopically signs were not seen. P. multocida were cultured back from all inflammatory foci.
Conclusion
As evident from the current study, it can be concluded that the use of combined treatment of ofloxacin antibiotic and grape seed extract, is very effective in increasing immune protection of rabbits against pasteurellosis.
Authors Contribution
Sawsan M.A. El-Sheikh, Azza A.A.Galal: Experimental design and critical revesion of the manuscript.
Fatma .A. Youssef and Haidi I.Mohamed: Experimental work and manuscript.
Amina .E.Dessouki: Histopathological investigations.
Conflict of interest
The authors declare that there is no conflict of interest.
References






