Advances in Animal and Veterinary Sciences
Research Article
A potential Use of Doppler Sonography for Evaluating Normal Hemodynamic Values of the Hepatic, Pancreatic and Splenic Vessels in Domestic Rabbits
Maher MA1*, Haithem A.M. Farghali2, Alaa H. Elsayed1 Ibrahim. A. Emam2, Elshymaa A. Abdelnaby3, Reem RT1
1Department of Anatomy and Embryology, faculty of Veterinary Medicine, Cairo University, Egypt; 2Department of Surgery, Anesthesiology and Radiology, faculty of Veterinary Medicine, Cairo University, Egypt; 3Department of Theriogenology, faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
Abstract | To determine Doppler hemodynamics reference values for hepatic artery, portal vein, pancreatic vessels and splenic vessels in healthy native breeds of rabbits (Oryctolagus cuniculus), this investigation was performed on twenty-one adult rabbits of both sexes. The first study concerned with the anatomical pattern of distribution of hepatic artery and portal vein of the liver; pancreatic arteries and veins; as well as splenic artery and vein. The second study included the Doppler sonography of these vessels. Doppler measurements showed that the PI, RI and S/D of hepatic artery (HA) were (0.87±0.11, 0.81±0.21, 0.61±0.03, 0.59±0.04, 4.65±0.12, 4.15±0.11), respectively. While the pancreatic artery (PA) Doppler indices which significantly (P=0.001) indicate the vascular perfusion were also measured as the PI, RI and S/D of PA were (1.05±0.33, 1.01±0.04, 0.79±0.12, 0.72±0.01, 3.35±0.21, 3.01±0.03), respectively but S/D did not give significant effect between sexes. All values of splenic vein (SV) were significantly higher in female than male cases in diameter and velocity (P=0.01, P=0.01), respectively. The mean diameter of SV was (6.01±0.18, 5.81±0.21) with a range and average (5.11-7.19, 5.89±0.63). While the splenic artery (SA) Doppler indices which significantly (P=0.002) indicated the vascular perfusion of the PI, RI and S/D of splenic artery (SA) were (1.02±0.35, 0.98±0.14, 0.77±0.03, 0.62±0.11, 3.62±0.12, 3.21±0.04), respectively. Conclusions and Clinical Relevance, Results provided hemodynamics reference values for hepatic, pancreatic and splenic vessels in domestic rabbits anesthetized with ketamine and xylazine. Values obtained from females were higher than that of males.
Keywords | Rabbits, Doppler, Hepatic artery, Portal vein, Pancreatic, Splenic vessels
Received | December 23, 2019; Accepted | March 03, 2020; Published | May 02, 2020
*Correspondence | Maher MA, Department of Anatomy and Embryology, faculty of Veterinary Medicine, Cairo University, Egypt; Email: [email protected]
Citation | Maher MA, Farghali HAM, Elsayed AH, Emam IA, Abdelnaby EA, Reem RT (2020). A potential use of doppler sonography for evaluating normal hemodynamic values of the hepatic, pancreatic and splenic vessels in domestic rabbits. Adv. Anim. Vet. Sci. 8(5): 506-518.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.5.506.518
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Maher et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The domestic rabbit is Considered as a companion animal. Hepatic disease had been described in pet rabbits (Yang et al., 2012) and the species had been widely used in splenic and pancreatic research (Su et al., 2006; Dimitrov et al., 2012). Ultrasonography (US) is the first choice of imaging modality used in the clinic in patients with diffuse liver disease, splenic abnormalities and chronic pancreatitis (Bonekamp et al., 2009; Dimcevski et al., 2013; Vancauwenberghe et al., 2015) or acute (Ripollé et al., 2010).The use of gray and color Doppler US in diagnosis and staging of chronic liver, splenic and pancreatic disease was based on the hypothesis that alteration of organ parenchyma and hemodynamics and these changes might reflect indirectly on the histological alterations (Gerstenmaier and Gibson, 2014).The combination of gray scale US and Doppler US improved the diagnostic accuracy and were essential for the diagnosis of cirrhosis or fibrosis (Cosar et al., 2005; Haktanir et al., 2005).
Doppler US was used to detect hemodynamic changes in patient’s liver (Popov et al., 2012), pancreas (Yoon et al., 2005) and spleen (Brusasco et al., 2018) with abnormalities that showed differences when compared to controls, the relationship between these parameters and the liver function was not been fully investigated. In the present study, we adopted an experimental native breeds of rabbit model to evaluate the normal hemodynamic values of the hepatic artery, portal vein, pancreatic vessels and splenic vessels by Doppler ultrasonography.
MATERIALS AND METHODS
This study was performed in accordance to the Institutional Animal Care and Use Committee (IACUC) of Faculty of Veterinary medicine, Cairo University (Vet. CU. IACUC) Vet CU1111201808. Twenty-one adult apparently healthy native breeds of Rabbits (Oryctolagus cuniculus) of both sexes and average body weight ranged from 2.500 – 3.500 kg were used. The first study concerned with the anatomical pattern of distribution of hepatic artery and portal vein of the liver; pancreatic arteries and veins; as well as splenic artery and vein. The second study included the Doppler sonography of these vessels in the rabbits.
Anatomical study
Fifteen rabbits were observed to be in good nutritional status. They were euthanized by lethal dose of Diazepam at 10 mg/kg introduced intravenously through the external jugular vein. Anatomical dissection was carried out via mid-laparotomy through the ventral abdominal wall at the linea alba from the caudal end of the sternum to the pubic bone. The abdominal wall was cut on both sides; cranially along the ribs and caudally along the inguinal region to expose the thoracic and abdominal viscera.
For studying hepatic, pancreatic and splenic vasculature; three specimens were injected by red-colored latex neoprene via the abdominal aorta to study the arterial ramification of the three organs, six were injected into the portal vein as it passed through the mesoduodenum by blue-colored latex neoprene to study the venous tributaries of the portal vein, other three specimens injected by red and blue-colored latex neoprene to observe both arterial and venous distribution of these organs. The organs were fixed by freezing or neutral buffered formalin and left overnight to be finally removed from the fixation media and carefully dissected. For cast study of the hepatic portal vein, after fixation, three specimens of those injected by blue-colored latex neoprene into the portal vein then transferred to concentrated Hcl solution and left for three days for cast preparation. Another three animals were used as specimens for the radiological examination of the hepatic portal vein distribution using the radiographic device (Fisher imaging, Chicago, USA). The animals were euthanized as mentioned before and injected by red lead oxide dissolved in turpentine oil into the major duodenal papilla to obtain x-ray films. All specimens were photographed using Olympus digital camera SP-600UZ 12 mega pixel. The anatomical nomenclature used in this study was in accordance with the Nomina Anatomica Veterinaria, 2017 (6th edition).
Doppler sonographic study
Animals and ultrasound scanning
Six (3 female+3 male) healthy native breeds of rabbits, 12 to 18 months of age and weighing 2.5 to 3.5 kg, were used. Rabbits were free of signs of liver, pancreatic or splenic disorders and were determined to be clinically normal by B-mode ultrasound. The rabbits were housed in stainless steel cages in a controlled environment, at temperatures of 24° to 26°C with 12 hours of light and 12 hours of dark/day. A commercial pellet diet and water were supplied ad libitum. Feed was withheld for a maximum of 6 hours before rabbits underwent Doppler examination to reduce abdominal distension from intestinal fill and intestinal movement which gave more color artifacts by Doppler color mode. The weight of each rabbit was recorded and Securing measures were taken to minimize defensive movements and to facilitate complete Doppler examination. For the views, rabbits were placed in dorsal recumbency over a table through which the ultrasound probe was brought from below and placed on shaved area on the liver, spleen and pancreas.
A pulsed-wave Doppler ultrasound scanner equipped with multifrequency 5-7.5 MHz (Abdelnaby et al., 2018) linear-array trans-rectal transducer (EXAGO, Echo Control Medical, France) was used for the examination of portal vein, hepatic artery, pancreatic artery and vein in addition to splenic artery and vein. The same operator performed all scans using the same Doppler ultrasound settings early in the morning. All examinations were performed with the following settings: frequency 5-7.5 MHz, depth 3 cm, acoustic power 80%, and gain 85 dB.
The electronic calipers of the ultrasound determined the largest diameter of portal vein, hepatic artery, splenic vein, splenic artery, pancreatic vein and pancreatic artery, while the color mode determined the direction of blood flow and the vascularization area within the vessel of interest (Abdelnaby and Abo El-Maaty, 2017a, 2017b; Abo El-Maaty and Abdelnaby, 2017), while the pulsed mode determine the spectral graph of known vessel.
Image analysis
Real-time B mode/color Doppler images were stored in the hard drive of the Doppler scanner then images and video clips were exported at the end of the experiment (Fouad et al., 2018) using a removable hard disk to a computer for colored area analysis.
Statistical analysis
Descriptive statistics are presented as Mean ± the standard error of the mean (SEM). All normal value of portal vein diameter, portal vein velocity, hepatic artery Doppler indices, hepatic artery peak velocity, pancreatic vein diameter, pancreatic vein velocity, pancreatic artery Doppler indices, pancreatic artery peak velocity, splenic vein diameter, splenic vein velocity, splenic artery Doppler indices and splenic artery peak velocity, effect of the sex either male or female was studied and blood flow estimated by colored area was evaluated using SPSS software (2007). Duncan’s Multiple Range test was used to differentiate between significant means at P<0.05.
RESULTS
Anatomical study
In rabbit, the celiac trunk (Figure 3, 4/2) divides into two main arteries; the splenic artery and a common trunk for the left gastric and the hepatic arteries (Figure 3, 4/3, 7, 8, 9). Whereas, the liver is supplied via intrahepatic branches of the hepatic artery (Figure 1), the spleen was supplied via the proper splenic branches of the splenic artery (Figures 3A, B; 4A, B/3, 4), while the pancreas was supplied via pancreatic branches of the splenic, the gastroduodenal, the cranial and caudal pancreaticoduodenal arteries (Figure 3). On the other hand, the venous drainage of these three organs were through venous tributaries of the portal vein which was an unpaired vein, receiving functional blood from stomach, spleen and pancreas and intestine except rectum and anus to deliver it to liver for detoxification and metabolism. In rabbit, the liver was functionally supplied via intrahepatic tributaries of the portal vein (Figure 2), while the spleen and the pancreas were drained via extrahepatic tributaries of the portal vein (Figure 4/24) which were the cranial mesenteric, gastro-splenic and gastroduodenal veins (Figure 4 /25, 26, 32).
Hepatic artery and portal vein
Close to the dorsal border of the rabbit liver, the hepatic artery (Figure 1/1) gave off A. gastroduodenal is (Figure 1a/1`), then after A. processus caudati (Figure 1/2) and continued distally on the cranial aspect of the portal vein, where it bifurcated ventral to the hepatic porta into the shorter right branch (Figure 1/3) and the longer left branch (Figure 1/4). A. lobi caudati (Figure 1/2) was a short vessel, emerged from the right aspect of the hepatic artery on a level with the caudate lobe isthmus, it penetrated the root of the caudate process and soon divided into two branches which followed the satellite ones of the portal vein to supply both surfaces of the caudate process. Ramus dexter (Figure 1/3); it proceeded ventrad with the right portal branch through the parenchyma of the right lobe towards the liver margin. Along its course, it gave off several branches (Figure 1/5) to the different portions of the lobe, in addition the cystic artery (Figure 1/6) for the gall bladder and a fairly large dorsolateral branch (Figure 1a/7) for the right dorsal part of the lobe. R. sinister (Figure 1/4); it accompanied the dorsal, ventral, cranial and caudal aspects of the left portal branch. At first, it gave off a dorsally directed omental branch (Figure 1/8) to spread through the papillary process of the caudate lobe, followed by the ventrally oriented artery of the quadrate lobe (Figure 1/9), which detached an accessory branch (Figure 1/10) for the right portion of the left medial lobe. The R. sinistri A. hepatica finally terminated into a left medial branch (Figure 1/11) for the greater portion of the left medial lobe and two to three left lateral branches. In the most common pattern, two relatively large Rr. sinistri laterales arose; a dorsal branch (Figure 1/12) and a common trunk (Figure1b/13,14) established by the union of the intermediate and ventral branches. In one examined specimen, the intermediate branch erupted by a common trunk with the dorsal one. In another investigated case, the three branches emerged separately from the terminal portion of the left hepatic branch (Figure 1a/12, 13, 14). In all examined specimens, the left lateral arterial branches accompanied their satellite portal venous branches and its subsequent ramifications in the left lateral lobe.
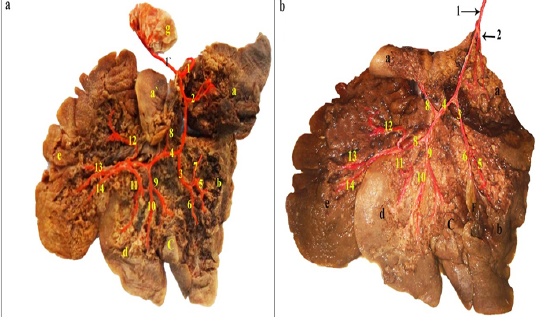
Figure 1: a and b: Visceral surface of the rabbit liver dissected- showing the intrahepatic branches of the hepatic artery- injected with colored latex neoprene.
a: Processus caudatus; lobus caudatus; a’. Processus papillaris, lobus caudatus; b: Lobus hepaticus dexter; c: Lobus quadratus; d: Lobus sinister medialis; e: Lobus sinister lateralis; f: Vesica fellea; g: Pylorus; 1: A. hepatica; 1’: A. gastroduodenalis; 2: A. processus caudati; 3: R. dexter; 4: R. sinister; 5: Rr. Dextri; 6: A. cystica; 7: A. dorsalis lateralis lobi dexter; 8: R. omentalis; 9: A. lobi quadrati; 10: R. accessorius lobi sinistri medialis; 11: R. lobi sinistri medialis; 12: R. dorsalis lobi sinistri lateralis; 13: R. intermedius lobi sinistri lateralis; 14: R. ventralis lobi sinistri lateralis.
The portal vein (Figure 2/1) divided on reaching the caudate process isthmus into a shorter right branch (Figure 2/2) and a longer left branch (Figure 2/3). The latter divided into a transverse part (Figure 2/7) and an umbilical part (Figure 2/8).
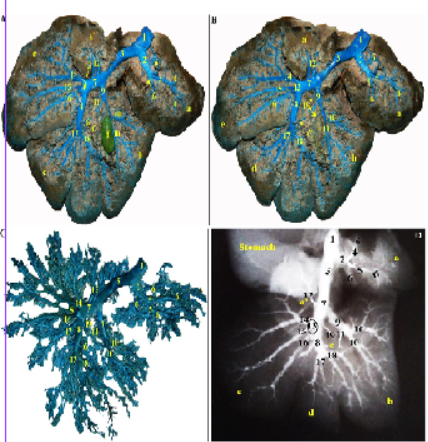
Figure 2: Photographs showing the intrahepatic branches of the portal vein in Rabbit. (A and B): Visceral surface of the rabbit liver – dissected- injected with colored latex neoprene. A. Gall bladder in situ (injected), B. Gall bladder was removed. (C) colored latex neoprene cast– caudal view. (D) Radiographic X-ray film.
a: Processus caudatus, lobus caudatus; a’: Processus papillaris, lobus caudatus; b: Lobus hepaticus dexter; c: Lobus quadratus; d: Lobus sinister medialis; e: Lobus sinister lateralis; f: Vesica fellea; 1: Venae portae; 2: R. dexter venae portae; 3: R. sinister venae portae; 4: R. dorsalis dexter; 5: R. ventralis dexter; 6: R. processus caudate; 7: R. sinister pars transversa; 8: R. sinister pars umbilicalis; 9: R. lobi dexter; 10: Rr. lobi dextri; 11: Rr. lobi quadrati; 12: Rr. Omentales; 13: Rr. lobi sinistri lateralis; 14: R. dorsalis lobi sinistri lateralis; 15: R. intermedius lobi sinistri lateralis; 16: R. ventralis lobi sinistri lateralis; 17: Rr. lobi sinistri medialis; 18: R. lobi quadrati; 19: Rr. quadrati dorsalis.
R. dexter venae portae (Figure 2/2) was fairly large, originated from the right ventral aspect of the portal vein. It penetrated the root of the caudate process of the caudate lobe and bifurcated after a short course into a dorsal (Figure 2/4) and a ventral branch (Figure 2/5). The parent venous branch and its subsequent divisions ramified into the parenchyma of the caudate process as caudate branches of the portal vein (Figure 2/6).
R. sinister venae portae (Figure 2/3) was the longest and the largest portal branch. It extended, beyond its separation from the right one, ventrolaterally to the left, at first as a transverse part (Figure 2/7) between the ventral aspects of the caudate lobe isthmus and the papillary process of the caudate lobe, dorsally and the large right lobe and the quadrate lobe, ventrally. On a level with the boundary between the quadrate and the left medial lobes, the parent left portal vein curved sharply as an umbilical part (Figure 2/8) which terminated abruptly just above the umbilical (round) notch. Along its course, the left branch of the portal vein detached R. lobi dexter, Rr. omentales, Rr. lobi sinistri lateralis and Rr. lobi sinistri medialis.
R. lobi dexter (Figure 2/9); originated from the ventral aspect of the transverse part of the left portal branch, proceeded ventrolaterally through the parenchyma of the right lobe, accompanied the cystic duct of the gall bladder. It detached 3-4 relatively large lateral branches and several small medial ones (Figure 2/10), ramified within the different portion of the right lobe, the gall bladder fossa as well as the dorsal part of the quadrate lobe (Figure 2/11).
Rr. omentales (Figure 2/12); were represented by two small veins erupted from the dorsal aspect of the transverse part of the portal vein and ramified into the parenchyma of the papillary process of the caudate lobe.
Rr. lobi sinistri lateralis (Figure 2A, B, D/13); four to five veins erupted either separately or by a common trunk from the convexity of the curve between the transverse and the umbilical parts of the left portal branch. They were differentiated into dorsal (Figure 1/14), intermediate (Figure 2/15), and ventral (Figure 2/16) branches. Each radiated and ramified into the respective portions of the left lateral lobe.
Rr. lobi sinistri medialis (Figure 2/17); five to six branches emanated from the lateral and ventral aspects of the umbilical part of the left portal branch, proceeded ventrolaterally to supply the parenchyma of the left medial lobe.
R. lobi quadrati; a fairly large quadrate branch extended ventrocaudally from the ventral end of pars umbilicalis and branched into the middle and ventral portions of the quadrate lobe, the gall bladder fossa as well as the ventral part of the right lobe (Figure 2/18). It must be noted that, several twigs emanated from the pars transversa venae portae and its divisions; the R. lobi dexter as well as the medial aspect of the pars umbilicalis venae portae and ramified into the dorsal and the intermediate parts of the quadrate lobe (Figure 2/11, 18, 19).
Pancreatic arteries and veins
The blood supply of the pancreas was categorized regarding the part of the organ being supplied; the cranial two thirds of the pancreatic right lobe was supplied
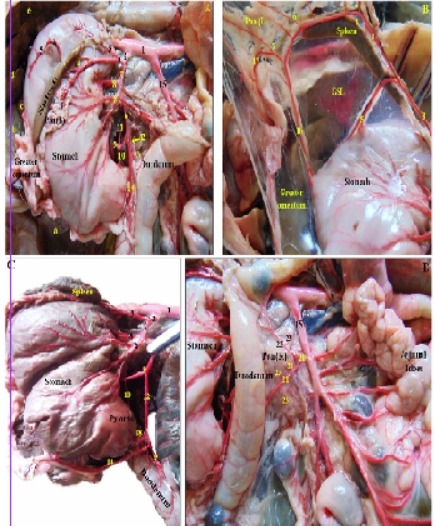
Figure 3: Photographs showing the blood supply of the pancreas and spleen in rabbit -dissected- injected with colored latex neoprene. (A): branches of the celiac trunk. (B): branches of the splenic artery. (C,D): Photographs showing the arterial supply of the body and right pancreatic lobe by branches of the gastroduodenal and caudal pancreaticoduodenal arteries.
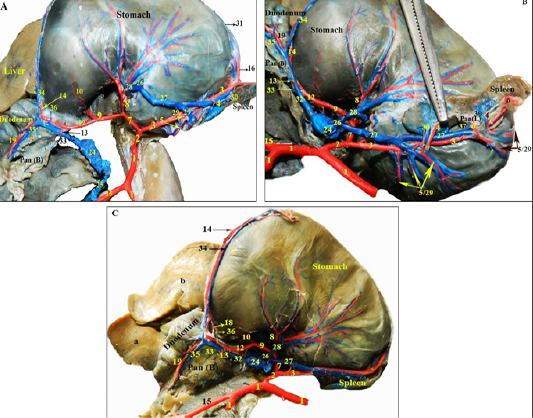
Figure 4: Photographs showing the branches of the extrahepatic tributaries of the portal vein draining the pancreas and spleen in rabbit, alongside their satellite arteries rabbit -dissected- injected with colored latex neoprene. (A): the extrahepatic tributaries of the portal vein, notice the gastrosplenic vein is cut and reflected. (B): the splenic vein and its branches. (C): branches of the gastroduodenal vein.
Legends for Figures 3 and 4: GSL: Gastrosplenic ligament; Pan (R): Right pancreatic lobe; Pan (B): Pancreatic body; Pan (L): Left pancreatic lobe. a: Processus caudatus; lobus caudatus; b: Lobus hepaticus dexter; c: Lobus quadratus; d: Lobus sinister medialis; e: Lobus sinister lateralis; 1: Abdominal aorta; 2: Celiac trunk; 3: Splenic artery; 4: Proper splenic arteries; 5: Short gastric arteries; 6: Pancreatic rami of 3; 7: Common trunk; 8: Left gastric arteries; 9: Hepatic artery; 10: Cranial pyloric artery; 11: Artery of the caudate lobe; 12: Gastroduodenal artery; 13: Pancreatic branch of 12; 14: Right gastroepiploic artery; 15: Cranial mesenteric trunk; 16: Left gastroepiploic artery; 17: Epiploic (Omental) branch; 18: Caudal pyloric artery; 19: Cranial pancreaticoduodenal artery; 20: Caudal pancreaticoduodenal artery; 21: Ante-mesenteric branch of 20; 22: Mesenteric branch of 20; 23: Pancreatic rami of 21 and 22; 24: Portal vein; 25: Cranial mesenteric vein; 26: Gastro-splenic vein; 27: Splenic vein; 28: Left gastric vein; 29: Short gastric veins; 30: Proper splenic veins; 31: Left gastroepiploic vein; 32: Gastroduodenal vein; 33: Pancreatic branch of 32; 34: Right gastroepiploic vein; 35: Cranial pancreaticoduodenal vein; 36: Right gastric vein; 37: Pancreatic veins of 27.
by fine pancreatic branches coming from the cranial pancreaticoduodenal artery of the gastroduodenal artery (Figures 3A, C; 4/12, 19), while the caudal third was supplied by the pancreatic branches of the ante-mesenteric artery of the caudal pancreaticoduodenal artery; a branch of the cranial mesenteric trunk (Figure 3D/15, 20, 21, 22, 23), those pancreatic branches eventually anastomosed within the vicinity of the right pancreatic lobe. The body and left lobe of the pancreas were supplied by 3-6 small pancreatic branches (rami pancreatici) arising from the splenic artery, 2mm from its celiac origin (Figures. 3A, B; 4A, B/3, 6), in addition to a direct pancreatic branch given off the gastroduodenal artery to the pancreatic body (Figure 3A, 4/12, 13).
The main tributaries of the pancreas of rabbit into the portal vein were the cranial pancreaticoduodenal vein of the gastroduodenal vein which drained the first two thirds of the pancreatic right lobe (Figure 4/32, 35), the caudal pancreaticoduodenal vein of the cranial mesenteric trunk draining the caudal third (Figure 4A/ 25), the gastroduodenal vein also received a pancreatic ramus to drain the pancreatic body (Figure 4/33), while the left pancreatic lobe was drained through pancreatic branches of the splenic vein (Figure 4B/37).
Splenic artery and vein
The spleen in rabbit was nourished mainly by a direct branch of the celiac trunk; the splenic artery which coursed to the left towards the splenic hilus in the gastrosplenic
Table 1: Normal parameters (mean ±SEM) of portal vein and hepatic artery with its doppler indices and systolic velocity in rabbit.
| Parameter | Mean ±SEM(F) | Mean ±SEM(M) | Range | Average | P-value |
| PV diameter(mm) |
5.43*±0.45 |
5.41*±0.32 |
5.21-6.99 | 5.62±0.68 | 0.04 |
| PV velocity(cm/s) |
38.21*±7.88 |
38.11*±3.18 |
32.11-43.65 | 38.8±0.312 | 0.001 |
| HA diameter(mm) |
4.12*±0.21 |
4.02*±0.01 |
4.03-5.89 | 4.49±0.01 | 0.001 |
| HA PI |
0.87*±0.11 |
0.81*±0.21 |
0.84-0.93 | 0.89±0.03 | 0.01 |
| HA RI |
0.61*±0.03 |
0.59*±0.04 |
0.55-0.81 | 0.65±0.02 | 0.01 |
| HA PSV(cm/s) |
34.12*±3.24 |
34.00*±2.36 |
30.54-38.25 | 35.12±2.65 | 0.002 |
| HA S/D | 4.65±0.12 | 4.15±0.11 | 4.22-5.11 | 4.72±0.44 | 0.09 |
SEM: standard error of mean; PV: portal vein; HA: hepatic artery; PI: pulstility index; RI: resistance index; PSV: peak systolic velocity; S/D: systolic /diastolic. * Means are significantly different at P<0.05.
Table 2: Normal parameters (mean ±SEM) of pancreatic vein (PV) and pancreatic artery (PA)with its Doppler indices and systolic velocity in rabbit.
| Parameter | Mean ±SEM(F) | Mean ±SEM(M) | Range | Average | P-value |
| PV diameter(mm) |
1.22*±0.01 |
1.18*±0.05 |
0.95-1.36 | 1.29±0.02 | 0.02 |
| PV velocity(cm/s) |
19.32*±2.35 |
17.11*±4.25 |
15.23-24.36 | 19.56±3.65 | 0.01 |
| PA diameter(mm) |
1.35*±0.15 |
1.24*±0.11 |
1.11-4.32 | 1.39±0.11 | 0.02 |
| PA PI |
1.05*±0.33 |
1.01*±0.04 |
0.99-1.19 | 1.11±0.21 | 0.001 |
| PA RI |
0.79*±0.12 |
0.72*±0.01 |
0.71-0.82 | 0.77±0.03 | 0.001 |
| PA PSV (cm/s) |
22.14*±4.25 |
20.36*±6.24 |
18.25-26.32 | 23.35±5.32 | 0.04 |
| PA S/D | 3.35±0.21 | 3.01±0.03 | 3.01-3.87 | 3.12±0.55 | 0.11 |
SEM: standard error of mean; PV: pancreatic vein; PA: pancreatic artery; PI: pulstility index; RI: resistance index; PSV: peak systolic velocity; S/D: systolic /diastolic. * Means are significantly different at P<0.05
Table 3: Normal parameters (mean ±SEM) of splenic vein (SV) and splenic artery (SA) with its Doppler indices and systolic velocity in rabbit.
| Parameter | Mean ±SEM(F) | Mean ±SEM(M) | Range | Average | P-value |
| SV diameter(mm) |
6.01*±0.18 |
5.81*±0.21 |
5.11-7.19 | 5.89±0.63 | 0.01 |
| SV velocity(cm/s) |
27.91*±6.95 |
26.43*±3.68 |
25.81-30.85 | 28.12±2.24 | 0.01 |
| SA diameter(mm) |
5.62*±0.22 |
5.14*±0.12 |
5.02-6.33 | 5.56±0.08 | 0.01 |
| SA PI |
1.02*±0.35 |
0.98*±0.14 |
0.89-1.19 | 0.99±0.36 | 0.002 |
| SA RI |
0.77*±0.03 |
0.62*±0.11 |
0.69-0.89 | 0.75±0.41 | 0.002 |
| SA PSV(cm/s) |
25.41*±2.56 |
24.02*±3.02 |
20.24-30.75 | 25.34 ±30.25 | 0.001 |
| SA S/D | 3.62±0.12 | 3.21±0.04 | 3.01-4.11 | 3.58±0.05 | 0.09 |
SEM: standard error of mean; SV: splenic vein; SA: splenic artery; PI: pulstility index; RI: resistance index; PSV: peak systolic velocity; S/D: systolic /diastolic. * Means are significantly different at P<0.05
ligament giving off 3-5 proper splenic branches to the spleen (Figures 3A, B; 4A, B/3, 4), short gastric arteries to the greater curvature of the stomach (Figures 3A, B; 4A, B/5) till it reached the base of spleen where it continued as left gastroepiploic artery (Figures 3B, 4A/16) which gave small epiploic (omental) branches to the greater omentum (Figures 3B, C/17) and continued to anastomose with the right gastroepiploic artery (Figures 3A, C, 4/14).
The portal vein received the splenic venous blood via the gastrosplenic tributary formed by the union of left gastric and splenic veins (Figure 4/26, 27, 28); the latter vein was about 5-6 cm long and receives proper splenic veins from the splenic hilus (Figure 4/30) as well as left gastroepiploic, pancreatic and short gastric veins (Figure 4/29, 31, 37).
Doppler sonographic study
From the detailed anatomical study and dissection, it was easy to know the exact vessel of interest and locate it using Doppler US, depending on its anatomic topography and surrounding landmarks. It was found that the major vessels supplying the liver and spleen; hepatic artery, portal vein, splenic artery and vein, were easily detected. However, the pancreatic parts were supplied by pancreatic branches of different origins, and it was noticed that only the cranial pancreaticoduodenal artery and vein supplying the cranial two thirds of the right pancreatic lobe were easily detected on Doppler US using the pylorus and first part of duodenum as a leading guide, while the small fine pancreatic branches of the splenic artery and vein supplying the left pancreatic lobe were difficult to be observed as they were concealed by the overlying spleen.
Hepatic artery (HA) and portal vein (PV) normal doppler parameter
The normal ranges of portal vein and hepatic artery are showed in Table 1, days of observation affected the normal diameter of portal vein significantly (P=0.04), and its velocity (P=0.001). The mean diameter of portal vein was (5.43±0.45, 5.41±0.32) with a range (5.21-6.99) in mm but its value increased in female than male rabbit .as well as its velocity measured with a normal value (38.21±7.88, 38.11±3.18) with a range (32.11-43.65) (Figure 5B).While the hepatic artery Doppler indices which significantly (P=0.01) indicated amount of blood flow were also measured as the PI, RI and S/D of hepatic artery were (0.87±0.11, 0.81±0.21, 0.61±0.03, 0.59±0.04, 4.65±0.12, 4.15±0.11), respectively. The hepatic artery diameter was significantly (P=0.001) higher in female (4.12±0.21) than in male rabbit (4.02±0.01) with a range (4.03-5.89) and average (4.49±0.01). The artery peak systolic velocity measures gave a normal value for rabbit and also affected significantly between gender (34.12±3.24, 34.00±2.36) with a range (30.54-38.25) and average (35.12±2.65) (Figure 5A).
Pancreatic artery and vein doppler parameters
The normal ranges of pancreatic vein and pancreatic artery were showed in Table 2, all values of pancreatic vein were significantly higher in female rabbit that male rabbit in diameter and velocity (P=0.02, P=0.01), respectively. The mean diameter of PV was (1.22±0.01, 1.18±0.05) with a range and average (0.95-1.36, 1.29±0.02) in mm but its value increased in female than male rabbit .as well as its velocity measured with a normal value (19.32±2.35, 17.11±4.25) with a range (15.23-24.36) and average (19.56±3.65) (Figure 5D).While the pancreatic artery (PA) Doppler indices which significantly (P=0.001) indicated the vascular perfusion were also measured as the PI, RI and S/D of PA were (1.05*±0.33, 1.01*±0.04, 0.79*±0.12, 0.72*±0.01, 3.35±0.21, 3.01±0.03), respectively but S/D gave no significant effect between sex. The PA diameter was higher in female (1.35*±0.15) than in male rabbit (1.24*±0.11). In addition to the artery peak systolic velocity measures gave a normal value for rabbit and also affected significantly (P=0.04) between gender (22.14*±4.25, 20.36*±6.24) with a range (18.25-26.32) and average (23.35±5.32) (Figure 5C).
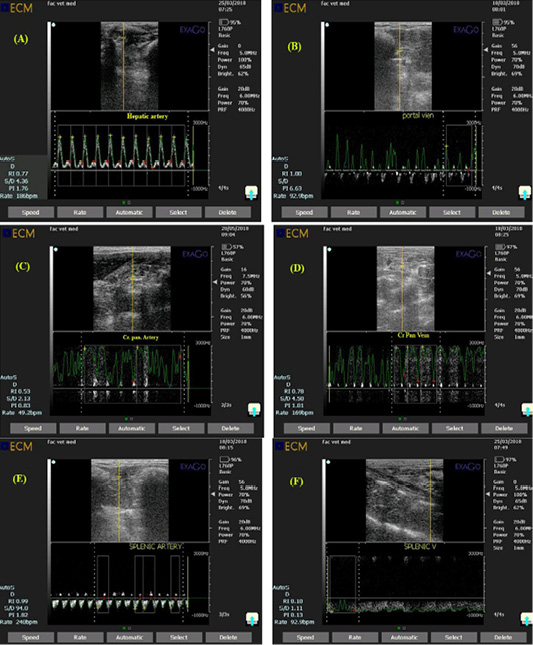
Figure 5: Ultrasonogram showing pulsed wave doppler mode of rabbit hepatic artery (A), portal vein (B), Cranial pancreaticoduodenal artery (C), Cranial pancreaticoduodenal vein (D), splenic artery (E) and Splenic vein (F) with a a gate opened in the known artery to measure amount of blood flow and a gate opened in the known vein to measure amount of blood drainage.
Splenic artery and vein doppler parameters
The normal ranges of splenic vein (SV) and splenic artery (SA) were showed in Table 3, all values of SV were significantly higher in female than male one in diameter and velocity (P=0.01, P=0.01), respectively. The mean diameter of SV was (6.01±0.18, 5.81±0.21) with a range and average (5.11-7.19, 5.89±0.63) in mm but its value increased in female than male rabbit. As well as its velocity measured with a normal value (27.91±6.95, 26.43±3.68) with a range (25.81-30.85) and average (5.89±0.63) (Figure 5F).While the splenic artery (SA) Doppler indices which significantly (P=0.002) indicate the vascular perfusion were also measured as the PI, RI and S/D of SA were (1.02±0.35, 0.98±0.14, 0.77±0.03, 0.62±0.11, 3.62±0.12, 3.21±0.04), respectively but S/D gave no significant effect between sex. The SA diameter in mm was significantly (P=0.01) higher in female (5.62±0.22) than in male rabbit (5.14±0.12). In addition to the artery peak systolic velocity measured gave a normal value for rabbit and also affected significantly (P=0.001) between gender (25.41±2.56, 24.02±3.02) with a range (20.24-30.75) and average (25.34 ±30.25) (Figure 5E).
DISCUSSION
Anatomical study
Our investigations revealed that the celiac artery of rabbit divided into two main arteries; the splenic artery and a common trunk for left gastric and hepatic arteries, where the latter gives rise to the gastroduodenal artery. A result which did not simulate the reports of (Abidu-Figueiredo et al., 2008) in rabbits that the celiac artery gave off splenic and left gastric arteries, the latter subdivided into hepatic and gastroduodenal arteries. While (Ozdemir et al., 2013) confirmed that the celiac artery in Chinchilla was divided into four main branches; the left gastric, hepatic, splenic and gastro-splenic arteries. Moreover, results observed by (Xavier-Silva et al., 2012) in cats and those of (Niza et al., 2003) in dogs revealed that both the celiac trifurcation into the splenic; left gastric and common hepatic arteries or bifurcation into the gastro-splenic and the common hepatic were the prominent morphological arrangement. But our observations in the current study revealed the bifurcation of celiac artery in rabbit into splenic artery and common trunk for left gastric artery and hepatic artery.
Our observation was similar to that reported by (Popesko et al., 2002; Abidu-Figueiredo et al., 2008; Ikegami et al., 2016) in rabbits that the splenic artery directed to the left towards the splenic hilus where it gave off 3-5 proper splenic branches and along its course in the gastro-splenic ligament, sends short gastric arteries to the stomach greater curvature till reaching the ventral end of spleen where it is continued as a left gastroepiploic artery which gives small branches to the greater omentum (epiploic branches) and continue to anastomose with the right gastroepiploic branch of the gastroduodenal artery. In agreement with the results of (Abidu-Figueiredo et al., 2005) in cats and (Ozdemir et al., 2013) in chinchilla rabbits, the splenic artery, after 2 mm from its origin, gave off small pancreatic branches to the body and left lobe of the pancreas, named pancreatic branches (rami pancreatici) which were 3-6 in number.
It was reported that the cranial pancreaticoduodenal artery was a branch of the gastroduodenal artery in porcupines (Atalar and Yilmaz, 2004), badgers (Yilmaz et al., 2004) and carnivores (Getty, 1975; Nickel et al., 1981). A result which is similar to our findings but disagreed with that found by (Ozdemir et al., 2013) in rabbits who confirmed that the cranial pancreaticoduodenal artery arose directly from the hepatic artery. Our observations agreed with the results of (Slatter, 2003; Lim et al., 2013) in dogs, that the right lobe of pancreas supplied by both the cranial pancreaticoduodenal artery for the body and proximal portion of right lobe while the caudal pancreaticoduodenal artery for the distal portion of right lobe of the pancreas.
According to the present investigations, the rabbit hepatic artery gave off the A. lobi caudati and then after divided into R. dexter and R sinister. A result which was in accordance with Ahmed et al. (1984), El-nady (1993), Alloush (1997), Barone (1997) in the same animal, but contradicted the findings of (Osman and Hagrass, 1986; Hagrass and Swielem, 1990; Abd El-hady, 2002) in ruminants as well as (Farag, 1990) in camel, who stated that the hepatic artery bifurcated directly into a right and a left branch.
Concerning the origin of the A. cystica from the R. dexter was recorded also in the rabbit (Alloush, 1997) and in swine and ruminants (Nickel et al., 1981). However, in carnivores, it was emanated either from the hepatic artery before its division (Anderson and Anderson, 1994) or from the left hepatic branch. A condition which was not observed in any of the examined liver specimens of rabbit.
Our study is in accordance with the observations of (Sharma et al., 2015) in dogs, that the Portal vein drained the blood of stomach, pancreas, spleen and all the intestine except for the anus and rectum.
Our investigations were in agreement with those of (Seo et al., 2001) in rabbits, who demonstrated that the most common anatomical pattern called “Conventional” which was the presence of an original portal vein which was formed mainly by the confluence of the mesenteric and the gastro-splenic veins. Our observations revealed that the extrahepatic tributaries of portal vein in studied rabbits were the gastro-splenic, gastroduodenal and mesenteric veins. While (Heath and House, 1970) in cats mentioned; the splenic, gastroduodenal, cranial and caudal mesenteric veins. However, (Ozudogru et al., 2005; Maher, 2015) in cats reported five tributaries; the splenic, gastroduodenal, right gastric, mesenteric and cystic veins. On the other hand, (Wingerd, 1985) in rabbits reported six tributaries; the cranial and caudal mesenteric veins, pancreaticoduodenal, gastroepiploic, gastro-splenic and coronary veins.
The splenic vein was formed by the union of the left gastroepiploic, pancreatic, proper splenic and short gastric veins and conjugates with the left gastric forming the gastro-splenic vein (Popesko et al., 2002; Abidu-Figueiredo et al., 2008) in rabbits. A result which was similar to our findings in the present study. The left gastroepiploic vein is found to be anastomosed with the right gastroepiploic vein in all studied rabbits. The right gastroepiploic vein draining blood to and was found as a tributary of the gastroduodenal vein. While, (Artico et al., 1998) in the domestic rabbits and cats reported a different result that the right gastroepiploic vein was a tributary to the splenic vein. A result which was not recorded in any of our specimens of rabbits.
Our present results in rabbits were similar to the observations of (Ozudogru et al., 2005) in cats that the drainage of the cranial two third of the right lobe of pancreas was done by the cranial pancreaticoduodenal vein of the gastroduodenal vein while the caudal third was drained by the caudal pancreaticoduodenal vein of the cranial mesenteric vein. In addition, the drainage of the left lobe was done by venous branches coming from the splenic vein.
According to Páramo et al. (2017), the rabbit intrahepatic portal vein was divided into three primary branches; R. processus caudati, R. dexter and R. sinister. A statement which was not accepted in the present investigation, from their directions and sizes, the portal vein bifurcated on a level with the caudate lobe isthmus into the shorter R. dexter, and the longer R. sinister. The former ramus supplied and distributed only into the caudate process of the caudate lobe and therefore, it could be favourly named also the R. processus caudati. While, the left ramus represented the continuation of the parent portal vein and branched into the remaining portions of the liver parenchyma.
According to Ozudogru et al. (2005) in van cat, and in canine (Zwingenberger et al., 2005; Ursic et al., 2007; Hall et al., 2015; Sharma et al., 2015), the right portal branch was distributed to the right lateral lobe as well as the caudate process of the caudate lobe, while the longer left portal branch branched into the right medial lobe and the remaining liver portions. The present study on the rabbit liver, recorded that the portal branch of the right lobe was erupted as the first branch of the left portal branch, simulating the previous observations in van cat and canine. In this respect, Rex (1888) named it as R. cysticus, while Elias and Petty (1953) termed it as R. centralis.
In the present investigation in rabbits’ livers, the left branch of the portal vein was differentiated into pars transversa and pars umbilicalis on the basis of the NAV (2017), and some relevant literatures (Ranjbar and Ghandiri, 2011), However, Sharma et al. (2015) divided it into transverse and oblique portions.
Doppler sonographic study
This study was aimed at assessing using Sonography the normal mean portal vein diameter and its velocity determination by sex. The study revealed a normal mean portal vein diameter of 5.43 mm ±0.45 SE in female and 5.41 mm ±0.32 SE in male with a variation between sex. In addition, the normal mean portal vein diameter seemed to have varied by sex. The normal mean portal vein diameter in our setting average (5.62±0.68) ranged from (5.21-6.99) in a human study done in Addis Ababa, Ethiopia the mean diameter (7.9 mm±2 SD) (Hawaz et al., 2012). And other other studies done in USA (11 mm±2 SD) (Weinreb et al., 1982), Nigeria (11.45 mm±1.49 SD) (Anakwue et al., 2009), and Kolkata (11.54 mm) (Lopamudra et al., 2011). In humans also, sex variation of the mean portal diameter was studied (Geleto et al., 2016; Ravi et al., 2011; Hawaz et al., 2012) and found that mean diameter of the portal vein did not vary by sex.
This study evaluated the normal portal vein velocity ranged (32.11-43.65). The mean velocity values obtained in this study for portal vein right branch flow also obtained in other studies of the main portal vein, in which the normal value was between in dog (Nyland and Fisher, 1990). The assessment of portal vein blood flow velocity was important, since the main portal vein was responsible for carrying, on average, 75% of the total blood received by the liver (Kantrowitz et al., 1989). In human contrast to our finding, tested 35 volunteers were tested and the normal value of portal venous flow and the blood flow velocity was assested (Brown et al., 1989), however, all other publications state that reduced blood flow and velocities dropping to less than 15 cm/s indicated portal hypertension and slowing of the blood flow in the portal venous bed increases the risk of thrombosis (Bernades et al., 1992; Iranpour et al., 2016).
While the hepatic artery Doppler indices in this study indicate the amount of blood flow were also measured as the PI, RI and S/D of hepatic artery ranged (0.84-0.93, 0.55-0.81, 4.22-5.11), respectively. In studies with human hypertension, the hepatic arterial pulsatility index was significantly higher in patients than in controls, which indicated lower vascularity (Iwao et al., 1997). In addition to the decrease in hepatic arterial RI (RI < 0.55) than a normal value due to increased diastolic flow is a more ominous finding than increased RI and is usually of concern for arterial complications. Decreased RI may occasionally be a transient finding on an immediate postoperative scan, which resolved in a few days. This was probably due to anastomotic edema in the postoperative period (Sanyal et al., 2014), in addition to the hepatic artery is a low-resistance systemic artery with low-resistance monophasic waveforms., in the case of portal hypertension or portal vein thrombosis, both of which decrease the portal venous flow within the liver, the arterial flow rate and velocity may increase as a compensatory response (McNaughton and Abu-Yousef, 2011). In addition to in cases suffered from portal vein thrombosis have been shown to have decreased hepatic arterial resistive indices, in some cases less than 0.50. increased hepatic arterial flow and velocity increase cause a decrease in the liver vascular index, which had been shown to have a high sensitivity and specificity in the diagnosis of cirrhosis and portal hypertension (Iwao et al., 1997).
In our study, the result showed that the mean diameter of cranial pancreaticoduodenal artery (PA) with a range (1.11-4.32) in mm. The divided arteries were mainly transverse pancreatic (TP), superior TP, and dorsal pancreatic (DP) arteries. The superior TP artery was observed in 24/38 specimens (63.2%) and ran along the superior ventral side of the head of the pancreas, therefore the surgeons need to be aware of the anatomy of the superior TP artery as well as a good knowledge of the vascular supply to the organ (Kimura et al., 2004).
The knowledge of pancreatic and splenic artery and vein vessel diameter and normal doppler indices were of great importance as chronic obstruction to venous return from splenic vein (SV) could give rise to a compartmentalized form of portal hypertension called Portal Hypertension (PH) (Wang et al., 2012). Etiology of PH is multifactorial and in cases of pancreatitis includes splenic vein thrombosis (Kokabi et al., 2010) or splenic vein compression from mass-forming chronic pancreatitis or a pseudocyst (Kokabi et al., 2010; Wang et al., 2012). Flow direction by doppler is still hepatopetal (Rösch et al., 1981) since primary pathology is not hepatic in origin.
In human Medicine, studies have shown that doppler ultrasound has increasingly contributed to the diagnosis and staging of pancreatic diseases, due to the significant increase in the sensitivity of this technique. Doppler was used to assess the intra and peri-pancreatic vasculature, such as the portal vein, splenic vessels, mesenteric vessels, and large vessels (Saftoiu, 2008; Vervloet and Martins, 2011).
To study in depth the determinants of splenic PI and RI, similar to a study analyzed the relationship with splenic arterial resistance was also analyzed (Bolognesi et al., 2012). In two previous studies, we demonstrated the relations between splenic PI values and portal venous resistance and portal pressure. On the other hand, the role of splenic arterial resistance in determining splenic PI values has not been defined. The significance of splenic PI as an index of splenic congestion is thus further confirmed and strengthened.
According to our knowledge, the present study was the first record of normal doppler indices values of liver, pancreas and spleen in rabbit, as there were no available literatures studying these values. There was a single research article which only investigated the characteristics of the vascularity of hepatic metastasis using Doppler US in rabbit (Du et al., 2003).
CONCLUSION
In native breeds of rabbits, the liver was supplied by the intrahepatic branches of the hepatic artery and functionally by the portal vein, the pancreas was supplied by pancreatic branches of the splenic, gastroduodenal arteries and cranial as well as caudal pancreaticoduodenal arteries and drained via their likewise satellite veins, while the spleen was supplied by the proper splenic arteries of the splenic branch of celiac trunk, however it was drained via the proper splenic vein of the gastrosplenic trunk.
In conclusion, the hepatic, splenic and pancreatic function reserve closely relates to the organ hemodynamics in the rabbit model. Doppler Ultrasound could be reliably used to assess the organ function reserve and hemodynamic changes which could help in prediction of any abnormalities of liver, spleen and pancreas in the future.
ACKNOWLEDGMENT
Great appreciation to professor Dr. Salah M. Hagrass professor of Anatomy and Embryology, faculty of Veterinary Medicine, Cairo University, for his continuous care, help and efforts. Without him, this manuscript could have not been.
Authors Contribution
Alaa Elsayed and Reem Tahoon collected the anatomical data and making review partition, Mohamed Maher and Alaa Elsayed anatomically dissected the specimens, photographed, written, discussed and making final editing. Haithem Farghali and Ibrahim Emam performed all surgical applications and El-Shaymaa Abdelnaby performed the ultrasonographic imaging.
Conflict of interest
Authors declared no conflict of interest.
REFERENCES






