Advances in Animal and Veterinary Sciences
Research Article
Pathogenetic Features Of The Metabolic Syndrome In Black-And-White Cattle (Metabolic Syndrome In Black-And-White Cattle)
Andrei Anatolyevich Rudenko¹, Yury Anatolyevich Vatnikov², Dana Aleksandrovna Korkots¹, Evgeny Vladimirovich Kulikov², Vladimir Petrovich Avdotin², Elena Anatolyevna Piven², Valentina Ivanovna Semenova², Anna Mikhailovna Orlova², Marina Ivanovna Shopinskaya², Nikolay Vladimirovich Sakhno³, Natalia Igorevna Troshina², Irina Anatolyevna Popova²*
¹Moscow State University of Food Production, Russia, Moscow, Volokolamskoe highway, 11, 125080; ²Peoples’ Friendship University of Russia (RUDN University). Moscow, st. Miklukho-Maklaya, 6, 117198; ³N.V. Parakhin Orel State Agrarian University, Russia, Orel, General Rodin, 69, 302019
Abstract | This article discusses the issues of changes in clinical, laboratory and instrumental parameters in black-and-white cows with increased body condition. The aim of the study is to establish the relationship between the increased fatness in black-and-white cows and clinical, tonometric, electrocardiographic, echocardiographic, biochemical parameters. The studies were carried out on clinically healthy black-and-white cows during the dry period 60 days before calving. All animals had one completed lactation. The experimental and control groups included cows with a milk yield for the previous lactation> 7500 kg, with a body condition score of> 4.25 points. The control group included clinically healthy cows with a body condition score of 3.0–3.5 points. Clinical, electrocardiographic, echocardiographic, and biochemical parameters were assessed. Increased body condition in black-and-white dairy cows leads to a significant increase in heart rate, systolic, diastolic and mean arterial pressure. In obese black-and-white cows, a significant increase in serum activity of alanine aminotransferase, lactate dehydrogenase and alkaline phosphatase. In black-and-white cows, with an increase in body condition, the cardiovascular system becomes more active, a tendency to the development of arterial hypertension, hepatopathy, and oxidative stress. Control of the body condition score, clinical, electrocardiographic, echocardiographic and biochemical parameters in dairy cows helps to prevent metabolic pathologies.
Keywords | Dairy cows, Obesity, Body condition score, Metabolic syndrome, Oxidative stress.
Received | July 22, 2021; Accepted | August 02, 2021; Published | December 01, 2021
*Correspondence | Irina Anatolyevna Popova, Peoples’ Friendship University of Russia (RUDN University). Moscow, st. Miklukho-Maklaya, 6, 117198; Email: [email protected]
Citation | Rudenko AA, Vatnikov YA, Korkots DA, Kulikov EV, Avdotin VP, Piven EA, Semenova VI, Orlova AM, Shopinskaya MI, Sakhno NV, Troshina NI, Popova IA (2022). Antimicrobial resistance and virulence genotyping of different salmonella serovars isolated from chickens in Egypt. Adv. Anim. Vet. Sci. 10(1): 85-93.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.1.85.93
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2022 Popova et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Protein, lipid, carbohydrate, metabolism and the functional state of internal organs (gastrointestinal tract, liver, cardiorespiratory and neuroendocrine systems, etc.), determine and regulate the basic biochemical, physiological processes in the organism (Esposito et al., 2014; Du et al., 2018; Rudenko et al., 2021). Nowadays cattle breeding suffers from enormous economic damage in case of metabolic disorders and diseases of internal organs due to the many factors, such as animal death, a decrease in productivity and reproductive function, the development of many infectious, invasive and non-infectious diseases. In this regard material costs for therapeutic and prophylactic activities increase (Putman et al., 2018; Zhang et al., 2018; Schuh et al., 2019).
The correlation between the live weight and the storage of adipose tissue in productive domestic animals is the main reason for the integrated visual system development. This system helps to assess the condition of animals in dairy farming (D’Occhio et al., 2019; Vailati-Riboni et al., 2020) and serves as a criterion for the feeding organization and allows to track the change in the ratio between body weight and body fat (Salcedo-Tacuma et al., 2020; Melendez et al., 2020).
Fatness in highly productive dairy cows has a significant effect on the productivity, milk composition, and the occurrence of some diseases (Dervishi et al., 2016; Singh et al., 2020). Both reduced and increased body condition has a negative effect on reproductive function in cows. It is also a risk factor for the metabolic disorders development (López-Gatius et al., 2003). Metabolic pathologies often occur in highly productive dairy cows and have a significant economic effect (Humer et al., 2018; Dong et al., 2019). The most dangerous and widespread metabolic disease in dairy cows is ketosis (Wu et al., 2020; Puppel et al., 2019). One of the factors in the ketosis development is overfeeding during the dry period. It causes a significant deposition of adipose tissue in the body and its active mobilization with the synthesis of ketone bodies in the early postpartum period (Steeneveld et al., 2020).
Determining the optimal condition of dairy cows during the calving interval and establishing the relationship between condition and production traits allows to control productive and reproductive qualities, optimize feeding conditions, and monitor the health of animals (Quigley et al., 2017; Neeland et al., 2019; Otten et al., 2018).
In this regard, the determination of the dairy cows condition and the study of relationship with clinical, biochemical and instrumental indicators of the health status of animals is of particular interest.
The aim of research is to establish the relationship between the increased fatness of black-and-white cows with clinical, tonometric, electrocardiographic, echocardiographic, and biochemical parameters.
Materials and methods
Ethical approval
The present studies were carried out in accordance with the State program of Peoples’ Friendship University of Russia (RUDN University). Selective research on animals was conducted after reciving approval of Bioethics comission SREC PFUR.
Study design
The study was conducted in accordance with the recommendations of the Helsinki Declaration and approved by the Ethics Committee of the Peoples’ Friendship University of Russia (EA1 / 1811, 05.10.2020). The studies were carried out on clinically healthy black-and-white cows on the farm of the Moscow region. The study was carried out on cows during the dry period 60 days before calving. All animals had one completed lactation. The experimental and control groups included cows with a milk yield for the previous lactation> 7500 kg. Animals were selected for the experimental group using inclusion and exclusion criteria, as well as the principle of analog pairs. Inclusion criteria: clinically healthy black-and-white cows, with a body condition score of> 4.25 points. Exclusion criteria: acute inflammatory processes (pericarditis, pleurisy, peritonitis, laminitis), infectious and parasitic diseases. The control group included clinically healthy cows with a body condition score of 3.0–3.5 points. Body condition was assessed visually and by palpation of the spine in the lumbar region, sacrum, and first caudal vertebrae. To assess the nutritional status in dairy cows, a 5-point system was used, with a step of ± 0.25 points (Chebel et al., 2018).
Clinical research methods
During the experiments and recording the results, the following research methods were used: thermometry, examination, palpation, percussion, tonometry, electrocardiography, echocardiography, serum chemistry.
Tonometry
Blood pressure was measured by high-resolution oscillometry on the tail artery using a PetMAP graphic II veterinary tonometer (Cardio Command Inc., USA). The cuff size corresponded to 40% of the tail circumference. 5-7 consecutive measurements of the blood pressure level were carried out, excluding abnormal results with a deviation of more than 10%, and the arithmetic mean value was calculated (Mossa et al., 2018).
Electrocardiographic method
Electrocardiograms in cows were recorded directly on the farm in the morning from 9.00 to 12.00 AM. The study was carried out during the resting period of the animal, to minimize the impact of any stress factors. To apply electrodes, the skin of the animal was preliminarily defatted with 95 ° ethanol, then its surface was moistened with Mediagel gel (Geltek-Medica, Russia) to optimize the contact and obtain a good quality electrocardiogram. A standard electrocardiogram was obtained using precordial leads according to a well-known method (Bonelli et al., 2018). The positive electrode was located at the level of the 5th left intercostal space caudal to the olecranon process, the negative electrode was located on the jugular groove in the lower 1/3 of the left neck, the third electrode was at the level of the left shoulder point. We used an EK1T-04 Midas apparatus (Russian Federation) (recording speed 50 mm / s, gain 1 mV / 10 mm).
Echocardiographic method
All echocardiographic studies were performed without sedation of animals in a standing position with the right thoracic limb laid forward (Hallowell et al., 2007). For the study, right-sided parasternal projections were used in the long and short axes of the left ventricle. A thick layer of Mediagel ultrasonic contact gel (Russian Federation) was used. The location of the sensor was 5–10 cm dorsal to the right olecranon process in 3–4 intercostal spaces. To optimize the quality of the ultrasound image, adjustment of depth, focal length, and signal amplification were used (total and in depth of scan). Images were obtained in the following projections: right cranial parasternal long-axis view to assess the outflow tract of the right ventricle, right caudal parasternal long-axis view to assess the left chambers of the heart and aortic root; right parasternal projection along the short axis at the levels of the aortic valve (assessment of the size of the aorta and left atrium), tendon chords of the mitral valve (assessment of the size of the interventricular septum, free wall and left ventricle). The following echocardiographic parameters were measured: the size of the left atrium (LA), the diameter of the aortic root (Ao), the size of the interventricular septum in diastole (IVSD) and systole (IVSS), the size of the free wall of the left ventricle in diastole (WLVD) and systole (WLVS), end-diastolic dimension (EDD), end-systolic dimension (ESD), left ventricular shortening fraction (FS). The study used a portable Mindray M7 ultrasound scanner with a P4-2s sector phased transducer (scanning frequency 1.3-4.7 MHz).
Biochemical methods
Blood sampling was carried out in the morning before feeding the animals. Blood samples were taken from the tail saphenous vein into vacuum tubes with a blood coagulation activator. Using a semi-automatic biochemical analyzer Stat Fax 1904 Plus (Awareness Technology, USA), the activity of alanine (ALT) and aspartic aminotransferase (AST) was determined in the blood serum by the Reitman-Frenkel method, lactate dehydrogenase (LDH) by the kinetic method, creatine phosphokinase (CK) method, alkaline phosphatase (ALP), urea - color reaction with diacetyl monoxime, creatinine - Jaffe reaction according to Popper method, cholesterol - enzymatic colorimetric method, triglycerides - turbidimetric method, total protein - biuret reaction, albumin - nephelometric biochemical method using (Olvex Diagnosticum, Russian Federation). The concentration of cardiac troponin in blood serum was determined by chemiluminescence immunoassay on microparticles on an Architect i2000 analyzer (Abbott, USA). The concentration of glucose and ketone bodies in the blood was measured using a FreeStyle Optimum Xceed glucose meter (Abbott Diabetes Care, China). The concentration of malondialdehyde in blood serum was determined in the test with thiobarbituric acid, ceruloplasmin - in the reaction with phenylenediamine dihydrochloride according to the following procedure (Stepanova et al., 2019).
Statistical methods
During the primary statistical processing, the normality of the distribution of the obtained digital data was preliminarily assessed using the Shapiro - Wilk test. When comparing two groups, the numerical indicators of which did not correspond to the normal distribution of signs, the nonparametric Mann-Whitney U-test was used. A 95% confidence interval (CI) was calculated. The difference between the indicators of cows in the experimental and control groups was considered significant at p <0.05. All calculations were performed on a personal computer using the statistical program STATISTICA 7.0 (StatSoft, USA).
Results and discussion
A sharp increase in body condition can cause a variety of hemodynamic changes that lead to both morphological and functional changes in the cardiovascular system. The high incidence of obesity leads to a high prevalence of cardiovascular complications, in particular, pulmonary hypertension syndrome associated with the pathology of the left heart chambers (Alpert et al., 2016). The problem of obesity in cattle is very relevant, since a sharp increase in body condition is a predictor of the metabolic multimorbid diseases development (Smith et al., 1997; Gärtner et al., 2019). The correlation between the detection and pathogenesis of cardiovascular complications in humans and high-yielding cows is similar (Krafsur et al., 2019).
In our study it was found that in clinically healthy cows of the control group the body condition score averaged 3.14 ± 0.04 points (95% CI 3.0 - 3.3), and body weight - 465.5 ± 4, 7 kg (95% CI 455.6 - 475.2), and in animals of the experimental group these parameters were significantly (p <0.001) 1.4 and 1.2 times higher, respectively (Table 1).
Table 1: Influence of the nutritional status in dairy cows on clinical indicators
| Value | Normal body condition (n=20) | Increased body condition (n=20) | ||
| M±m | 95% CI | M±m | 95% CI | |
| Body condition score | 3,14±0,04 | 3,0– 3,3 | 4,41±0,05*** | 4,2 – 4,6 |
| Body weight, kg | 465,5±4,7 | 455,6 – 475,2 | 566,5±6,8*** | 552,2 – 580,7 |
|
Temperature, ºС |
38,4±0,1 |
38,2 – 38,5 |
38,4±0,1 | 38,3 – 38,6 |
| Pulse, beats / min | 72,5±1,5 | 69,5 – 80,3 | 79,6±1,4 ** | 76,6 – 82,6 |
| Breathing, units / min | 16,3±0,8 | 14,6 – 17,9 | 21,1±1,0 ** | 18,9 – 23,1 |
| Systolic blood pressure, mmHg | 113,7±1,5 | 131,0 – 125,2 | 131,0±2,8*** |
125,2 – 136,8 |
| Diastolic blood pressure, mm Hg | 49,8±1,9 | 45,8 – 53,7 | 74,6±2,2*** |
70,0 – 79,2 |
Note: * (p <0.05), ** (p <0.01); *** (p <0.001) - the reliability of the difference between the indicators of the group of animals with obesity and clinically healthy (Mann-Whitney test), CI - confidence interval
Table 2: Influence of the nutritional status on electrocardiographic parameters
| Value | Normal body condition (n=20) | Increased body condition (n=20) | ||
| M±m | 95% CI | M±m | 95% CI | |
| P, ms | 78,4±1,9 | 74,4 – 82,3 | 78,4±2,0 | 74,2 – 82,5 |
| PQ, ms | 167,0±4,6 | 157,5 – 176,5 | 144,4±4,2*** | 135,6 – 153,1 |
| QRS, ms | 73,7±2,6 | 68,2 –79,1 | 70,5±2,0 | 66,4 – 74,6 |
| QT, ms | 350,3±8,8 | 331,9 – 368,6 | 320,8±11,8 | 296,1 – 345,5 |
| PII, mV | 0,22±0,01 |
0,15 – 0,19 |
0,15±0,01*** | 0,12 – 0,17 |
| SII, mV | -0,64±0,03 | -0,72 – (-0,58) | -0,38±0,06*** | -0,49 – (-0,26) |
| TII, mV | 0,34±0,03 | 0,27 – 0,41 | 0,19±0,03*** | 0,14 – 0,25 |
| ST, mV | 0,03±0,02 | -0,04 – 0,04 | -0,03±0,02 |
-0,10 – 0,01 |
Note: * (p <0.05), ** (p <0.01); *** (p <0.001) - the reliability of the difference between the indicators of the group of obese and clinically healthy animals (Mann-Whitney test).
Table 3: Influence of the nutritional status on echocardiographic parameters
| Value | Normal body condition (n=20) | Increased body condition (n=20) | ||
| M±m | 95% CI | M±m | 95% CI | |
| LA, cm | 7,5±0,2 | 7,2 – 7,8 | 7,7±0,2 | 6,7 – 8,0 |
| Ао, cm | 6,0±0,1 | 5,8 – 6,1 | 6,1±0,1 | 7,2 – 7,9 |
|
LA/Ао, un |
1,26±0,02 |
1,21 – 1,31 |
1,22±0,03 | 1,16 – 1,28 |
| IVSD, cm | 1,77±0,04 | 1,69 – 1,85 | 2,01±0,04** | 1,92 – 2,09 |
| IVSS, cm | 2,83±0,05 | 2,72 – 2,93 | 2,92±0,04 | 2,84 – 3,01 |
| WLVD, cm | 1,97±0,05 | 1,86 – 2,71 | 2,03±0,04 |
1,94 – 2,11 |
| WLVS, cm | 2,87±0,03 | 2,79 – 2,94 | 3,11±0,03** | 3,03 – 3,18 |
| EDD, cm | 7,73±0,13 | 7,45 – 7,99 | 7,90±0,13 | 7,62 – 8,17 |
| ESD, cm | 4,98±0,12 | 4,74 – 5,23 | 5,19±0,10 | 4,99 – 5,39 |
|
FS, % |
35,5±1,2 | 33,0 – 37,9 | 33,9±1,3 |
31,1 – 36,7 |
Note: * (p <0.05), ** (p <0.01); *** (p <0.001) - the reliability of the difference between the indicators of the group of obese and clinically healthy animals (Mann-Whitney test).
Table 4: Influence of the nutritional status on the biochemical parameters
| Value | Normal body condition (n=20) | Increased body condition (n=20) | ||
| M±m |
95% ДИ |
M±m |
95% ДИ |
|
| ALT, U / l | 44,7±4,0 | 36,2 – 53,1 | 77,7±4,9*** | 67,4 – 87,9 |
| AST, U / l | 110,9±4,4 | 101,8 – 120,1 | 126,8±6,3 | 113,5 – 140,1 |
| LDH, U / l | 246,2±17,3 | 209,9 – 282,4 | 328,9±18,2*** | 290,9 – 366,8 |
| CK, U / l | 93,6±6,6 | 79,8 – 107,3 | 128,2±11,1 | 104,9 – 151,4 |
| ALP, U / l | 120,0±6,6 |
106,1 – 133,9 |
209,9±20,8* | 166,4 – 253,4 |
| Troponin, ng / ml | 0,04±0,01 | 0,01 – 0,06 | 0,07±0,02 | 0,03 – 0,10 |
| Urea, mmol / l | 4,1±0,3 | 3,5 – 4,7 | 3,7±0,3 | 3,0 – 4,4 |
|
Creatinine, μmol / l |
126,1±9,2 | 106,8 – 145,4 | 131,7±8,1 |
114,7 – 148,7 |
| Glucose, mmol / l | 2,7±0,1 | 2,4 – 2,9 | 2,6±0,1 | 2,4 – 2,8 |
| Ketone bodies, mmol / l | 0,72±0,04 | 0,65 – 0,80 | 0,69±0,07 | 0,55 – 0,84 |
| Cholesterol, mmol / l | 2,9±0,2 | 2,6 – 3,2 | 4,6±0,2*** | 4,2 – 5,1 |
| Triglycerides, mmol / l | 0,8±0,1 | 0,7 – 0,9 | 1,6±0,1*** | 1,3 – 1,9 |
| Total protein, g / l | 73,7±1,2 | 71,2 – 76,2 | 75,9±1,1 | 73,7 – 78,1 |
| Albumin, g / l | 31,4±0,7 | 30,2– 32,6 | 32,1±0,6 | 30,7 – 33,4 |
|
Malonic dialdehyde, μmol / l |
1,3±0,04 | 1,2 – 1,4 | 2,3±0,13*** | 2,1 – 2,6 |
| Ceruloplasmin, mmol / l | 1,5±0,1 | 1,4 – 1,7 | 2,7±0,1*** |
2,4 – 3,0 |
Note: * (p <0.05), ** (p <0.01); *** (p <0.001) - the reliability of the difference between the indicators of the group of obese and clinically healthy animals (Mann-Whitney test).
In human medicine, obesity cardiomyopathy is described. People with obesity may experience systolic and diastolic dysfunction, myocardial hypertrophy, and arterial hypertension syndrome (Alpert, 2001). In human medicine, metabolic syndrome is well described as a combination of obesity, insulin resistance, dyslipidemia, and arterial hypertension (Samson and Garber, 2014; Nishida and Otsu, 2017; Saklayen, 2018; Nakamura and Sadoshima, 2020). In veterinary medicine, metabolic syndrome has not been adequately studied (Morgan, 2015; Krafsur, 2019).
As presented in the data of Table 1, in highly productive clinically healthy black-and-white cows, some changes were noted. The pulse rate (by 1.1 times; p <0.01), the respiratory rate (in 1.29 times; p <0.01), the level of systolic (1.15 times; p <0.001), diastolic (1.49 times; p <0.001) and mean arterial pressure (1.49 times; p <0.001) significantly increased compared to cows with a normal body condition score. It should be noted that between groups of cows there were no significant changes in rectal body temperature, pulse rate and respiratory rate at rest. These changes are obviously associated with the activation of the neurohumoral system. Neurohumoral, immune-inflammatory and metabolic pathophysiological mechanisms can contribute to the obesity and initiate the chronic heart failure syndrome development (Chernigova et al., 2019; Vatnikov et al., 2019; Vatnikov et al., 2020). Obesity is accompanied by the arterial hypertension development in dogs (Tropf et al., 2017), cats (Zahrazadeh et al., 2018), pigs (Zhang et al., 2015), horses (Patterson et al., 2020) and humans (Fantin et al., 2019; Seravalle and Grassi, 2017). Obesity in dogs is accompanied by a significant change in metabolism: an increase in the ratio of serum insulin to glucose concentration, dyslipidemia, increased cholesterol, triglycerides, high density lipoproteins (Tropf et al., 2017).
The change in mean arterial blood pressure in cows depending on the nutritional status is shown in Figure 1.
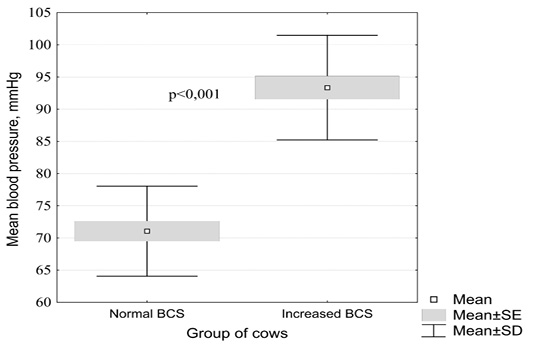
Figure 1: Influence of the nutritional status in clinically healthy cattle on mean arterial blood pressure (by the Mann-Whitney test)
In clinically healthy black-and-white cows with a normal body condition score, the mean arterial blood pressure averaged 71.1 ± 1.6 mm Hg. (95% CI 67.8 - 74.3), compared with animals with increased fatness - 93.4 ± 1.8 mm. rt. Art. (95% CI 89.5 - 97.2). These changes in cows from different experimental groups were found to be statistically significant (p <0.001).
Table 2 presents the influence of the nutritional status in black-and-white cows on the electrocardiographic parameters.
It should be noted that we have not established clinically significant changes in the function of excitability, conductivity, automatism, repolarization in animals of different experimental groups. All cows had a regular sinus rhythm with a normal heart rate (sinus normocardia). However, it was found that in clinically healthy cows with increased body condition, compared with those with normal body condition score values, there was a significant shortening of the PQ interval (by 1.16 times; p <0.001) and a decrease in the voltage of the P wave (by 1.46 times; p <0.001), T (1.78 times; p <0.001), S (1.68 times; p <0.001) in standard lead II. These changes occurred in the range of reference norms for clinically healthy high-yielding dairy cows.
The results of studies on the effect of body weight condition in clinically healthy black-and-white cows on baseline echocardiographic parameters are shown in Table 3.
When analyzing the results of echocardiographic studies in clinically healthy black-and-white cows, clinically significant changes were nor found (Table 3). In cows with an increased body condition score, compared with animals with normal body condition, there was a significant increase in IVSD (by 1.13 times; p <0.01), LVS (by 1.1 times; p <0.01). However, these changes were not accompanied by hemodynamic disturbances and remodeling of the heart chambers. These changes in heart rate can be explained by the activation of the sympathetic part of the autonomic nervous system, which is also noted in cows wiht other metabolic diseases (Aoki et al., 2020). The decrease in the voltage of the electrocardiogram teeth in the experimental group of cows is associated with a decrease in electrical resistance during the deposition of adipose tissue in the body. Attention should be focused on the fact that the functions of excitability, conduction, automatism, repolarization of the myocardium are not impaired in black-and-white cows with obesity. By the method of echocardiographic study in cows with an increased body condition score, a significant increase in the diastolic size of the interventricular septum and the systolic size of the free wall of the left ventricle was established. Similar echocardiographic changes have been described in the development of obesity in dogs (Tropf et al., 2017).
The study of the influence of the fatness degree in cows on the biochemical parameters in black-and-white cows is given in Table 4.
From the data presented in Table 4, it can be seen that in black-and-white cows with an increased body condition, in comparison with those of animals with a normal body condition, a significant increase in the serum activity of alanine aminotransferase (by 1.73 times; p <0.001) , lactate dehydrogenase (1.34 times; p <0.001) and alkaline phosphatase (1.75 times; p <0.05), cholesterol concentration (1.58 times; p <0.001), triglycerides (2.0 times; p <0.001), malondialdehyde (1.77 times; p <0.001) and ceruloplasmin (1.8 times; p <0.001) was established.
These changes indicate a tendency towards the development of hepatocyte cytolysis syndrome and extrahepatic cholestasis in cows with increased body condition, as well as lipid metabolism disorders. Disturbances of lipid metabolism in dairy cows associated with increased body condition have also been described in other studies (Wu et al., 2020; Karis et al., 2020).
Highly productive cows with high body condition parameters are susceptible to the development of oxidative stress (Zahrazadeh et al., 2018; Teng et al., 2018). In our study, a significant increase in the concentration of malondialdehyde and ceruloplasmin was found in the blood serum of cows with increased body condition. Another study showed an increase in the concentration of malondialdehyde in the serum of cows with an increase in body condition at the beginning of lactation, which was a predictor of the development of subclinical ketosis (Senoh et al., 2019). Changes in the concentration of cardiac troponin in cows with an increase in body condition were not observed, which indicates the absence of the syndrome of cytolysis of cardiomyocytes.
Thus, the use of the body condition score, clinical and instrumental indicators, in combination with the determination of hematological parameters in practice, allows to monitor the health of dairy cows throughout the entire production cycle, as well as preventing the occurrence of pathological conditions in the tissues of the myocardium, liver and other internal organs and complications in the form of metabolic pathology.
Conclusion
The increased body condition in dairy cows of the black-and-white breed leads to a significant increase in heart rate, respiration rate, systolic, diastolic and mean arterial pressure. At the same time, there are no significant changes in electrocardiographic indicators. In cows, with an increase in body condition, sinus tachycardia develops, which leads to a significant decrease in the time of atrioventricular conduction (PQ interval). Cows with an increased body condition score showed a significant increase in the diastolic size of the interventricular septum and the systolic size of the free wall of the left ventricle. In obese black-and-white cows, a significant increase in the serum activity of alanine aminotransferase, lactate dehydrogenase and alkaline phosphatase, the concentration of cholesterol, triglycerides, malondialdehyde and ceruloplasmin was established. Control of the body condition score, clinical, electrocardiographic, echocardiographic and biochemical parameters in dairy cows makes it possible to prevent pathologies of metabolism and internal organs.
Acknowledgements
This paper was financially supported by the Ministry of Education and Science of the Russian Federation on the program to improve the competitiveness of Peoples’ Friendship University of Russia (RUDN University) among the world’s leading research and education centers in the 2016-2020.
Conflict of interest
We declare that authors have no competing interests.
Authors contribution
The authors contributed equally.
References






