Journal of Animal Health and Production
Research Article
Uterine Luminal Fluid Proteins in Buffalo During Follicular and Luteal Stages and Early Pregnancy
Devender Kumar, Govind Narayan Purohit*
Department of Veterinary Gynecology and Obstetrics, College of Veterinary and Animal Sciences,, Rajasthan University of Veterinary and Animal Sciences, Bikaner Rajasthan India 334001.
Abstract | In order to evaluate the changes in uterine luminal fluid proteins during different reproductive stages abattoir derived buffalo genital tracts were classified into proestrus (n=7), estrus (n=7), luteal phase (n=8) and early pregnancy (n=7). The genitalia were dissected and uterine fluid samples were collected in eppendorf tubes. SDS-PAGE analysis was performed on the uterine fluid samples and molecular weights (MWs) of protein bands were estimated by comparing their migration rates on the gel with those of protein markers with known MWs. A total of 24 different protein bands were observed corresponding to MWs varying from 10 to 210 kilodaltons (kDa) during different phases of reproduction with some proteins being specific to some stage of reproduction. Proteins with MWs between 90 and 160 kDa were not found in any of the samples. During proestrus, proteins with MWs between 75-80 kDa were absent whereas they were present during all other stages studied (estrus, luteal stage and early pregnancy). Proteins with high MWs (205-210 kDa) were observed during estrus only. Proteins with MWs between 10-35 kDa were specific to luteal phase and early pregnancy and proteins with MWs 15-20 kDa and 40-45 kDa were specific to pregnancy. It was concluded that the proteins in the uterine luminal fluid change during different reproductive stages in buffalo with low MWs proteins being common during luteal phase and pregnancy and high MWs proteins common during follicular phase.
Keywords | Buffalo, Uterine luminal proteins, Estrus, Pregnancy, Luteal phase.
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | March 05, 2018; Accepted | March 25, 2018; Published | March 30, 2018
*Correspondence | Govind Narayan Purohit, Department of Veterinary Gynecology and Obstetrics, College of Veterinary and Animal Sciences, Rajasthan University of Veterinary and Animal Sciences, Bikaner Rajasthan India 334001; Email: gnpobs@gmail.com
Citation | Kumar D, Purohit GN (2018). Uterine luminal fluid proteins in buffalo during follicular and luteal stages and early pregnancy. J. Anim. Health Prod. 6(1): 41-46.
DOI | http://dx.doi.org/10.17582/journal.jahp/2018/6.1.41.46
ISSN | 2308-2801
Copyright © 2018 Kumar and Purohit. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Uterine luminal fluid proteins are altered during various reproductive phases of many domestic animal species including cattle, horses and pigs. Appreciable changes in the uterine luminal fluid proteins have been recorded during the estrous cycle in mares (Martin and James, 2013), cows (Alavi-Shoustari et al., 2006; Alavi-Shoustari et al., 2008; Alavi-Shoustari et al., 2014) and pigs (Kayser et al., 2006). Significant changes in the uterine luminal proteins have been recorded during the pre-implantation period in pigs (Szafranska and Panasiewicz, 2002), ewes (Koch et al., 2010) and cattle (Forde et al., 2014).
Most uterine luminal proteins are of blood serum origin but uterine endometrium synthesized proteins also contribute significant proportions of proteins during early pregnancy (Forde et al., 2014). There are two species which have been studied more thoroughly and from which uterine proteins (i.e. proteins not found in the serum of the same animal) have been isolated. These are the rabbit and the pig, from which, respectively, uteroglobin and a phosphatase (Wynn and Jollie, 2013) have been purified.
Uterine proteins are shown to change quantitatively and qualitatively during the estrous cycle in pig (Skowronski, 2010). It has long been believed that there are quantitative and qualitative changes in uterine proteins at the time of implantation (Filant and Spencer, 2014). Progesterone is the main hormone that controls the uterine protein secretion (Bergeron, 2000) although many other interactions are involved.
In all species so far investigated, including man, ovulation is associated with the production of a voluminous, protein-rich uterine secretion of low viscosity (Bergeron, 2000). The physical function of these uterine fluids is to dilate the uterine lumen and thereby create a passage through which the spermatozoa can progress towards the utero-tubal junction.
Uterine proteins are considered to be signaling molecules for pregnancy recognition (Bazer, 2013). Pregnancy-specific proteins were found in bovine uterine flushing’s between Days 7 and 35 and from Day 15 of pregnancy onwards (Bazer, 2013). The initiation of implantation is associated with the presence of characteristic non-plasma proteins in the uterine lumen of many mammalian species (Raheem, 2017).
Endometrial secretions act as primary regulators of conceptus survival, growth and development during pregnancy (Gray et al., 2002; Spencer and Gray, 2006). It has been proposed that ewes exhibit recurrent early pregnancy loss due to inadequate conceptus elongation, the absence of specific components of uterine luminal fluid (ULF) derived from the endometrial glands and likely luminal epithelia (Gray et al., 2002).
Interferon- τ is major product from ovine and bovine conceptus during the period before the embryonic trophoblast makes firm attachment to the uterine wall and begins to form the placenta. It is secreted from Day 10 to 24 of gestation and peaks at Day 17 (Roberts et al., 2007; Bazer, 2013). It is glycoprotein in nature possessing a molecular weight of 20-24 kDa with 172 amino acids and regarded as the first in the series of signals from the conceptus required for pregnancy maintenance (Chelmonska-Soyta, 2002).
Uterine luminal proteins have been isolated in a limited number of studies in buffaloes during the follicular and luteal phases of the estrous cycle (Ramadan and Hassan, 1999; Roy et al., 2006) and early pregnancy (Balhara et al., 2014). The relationship of the transcriptome profiles of buffalo embryos and their survival was shown in a recent study (Strazullo et al., 2014). The present study examined the uterine luminal proteins during the luteal and follicular stages of pregnancy and early pregnancy in abattoir derived buffalo genitalia.
Materials and Methods
Collection of Samples
The study was undertaken at the Department of Veterinary Gynecology and Obstetrics, Veterinary College Bikaner utilizing abattoir derived buffalo genitalia. The complete genitalia were collected immediately after slaughter and were brought to the laboratory in a thermo-cool box with ice packs. Approximately 28 genital organs without any gross pathology and at the desired stage of reproduction (follicular, luteal and early pregnancy) were processed further.
Screening Criteria for Selection of Genital Organs
The collected genitalia were examined in the laboratory and classified as follicular/luteal stage or early pregnancy. For determination of the stage of estrous cycle (follicular/luteal) the presence/absence of CL, color, size and consistency of CL and presence/absence of follicles on the surface of the ovary was utilized as a criterion as described previously (Roy et al., 2006).
Briefly, the tracts were classified into follicular and luteal phase based on the corpus luteum morphology and presence of follicles. All stages of early to late luteal phase were included in the luteal phase in this study. Follicular phase included genitalia in proestrus and estrus phase.
A. Proestrus phase was characterized by the presence of a regressing CL with a developing follicle and appearance of rughae on uterine horn.
B. Estrus phase was characterized not only by the presence of regressed CL with no vasculature, cream color and hard texture in cut surface but also with at least one 5-10 mm or above diameter follicle and appearance of rughae on uterine horn.
C. Luteal phase was characterized by red to brown early developed CL.
D. Early pregnancy was considered when a conceptus was visible in the uterus at late luteal phase as mentioned for cattle (Forde et al., 2014).
Collection of Uterine Luminal Fluid
The uterine fluid was collected by excision of the uterine horns followed by gentle scraping of the endometrium by a curette and collecting the fluid in a 2 mL Eppendorf tube as mentioned previously (Alavi-Shoustari et al., 2006).
Experimental Procedure
The organs were divided into 4 groups (proestrus, estrus, luteal and early pregnant animal organs). The uterine luminal fluid was collected in Eppendorf tubes and immediately processed for SDS-PAGE analysis of uterine lumen proteins.
SDS-PAGE Analysis
For qualitative estimation of proteins the uterine luminal fluid was processed as per method described previously (Alavi-Shoushtari et al., 2008) with some modification using commercially available kits (Hi-Media, India).
Analysis of Results
The results were analyzeded by comparing the bright band with protein ladder after taking the image of destained gel by Gel-doc and read on a computer (Figure 1). By plotting the graph of known molecular weights of protein bands in the ladder a standard graph was prepared. The band travel distance of samples was interpolated on the graph manually to calculate the molecular weights of protein under study.
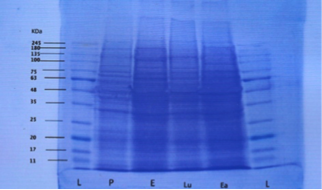
Figure 1: Destained gel showing protein band fractions during different phases (L=ladder, P=proestrus, E=estrus, Lu=luteal phase and Ea=early pregnancy
Results
Uterine Fluid Proteins During Follicular Phase of the Estrous Cycle
Both proestrus and estrus phase samples are described as follicular phase. The mean molecular weight of uterine fluid proteins during pro-estrus were 38.14±0.26, 63±0.90, 69.71±1.14, 88.42±3.13 and 160.28±2.53 kilodalton (kDa) respectively. Protein bands with molecular weights between 75-80 kDa were absent during proestrus.
The mean molecular weights of uterine luminal fluid proteins during estrus were 36.57±0.84, 65±1.34, 75.14±1.35, 85.42±1.16, 90±2.05 and 207.28±6.65 kDa. High molecular weight proteins (mean 205-210kDa) were estrus specific and appeared during estrus in comparison to proteins of molecular weights between 160-165 kDa during proestrus.
Proteins with molecular weight above 245 kDa could not be evaluated in the present study because standard ladder used was up to 245 kDa. There were two protein bands observed during estrus with mean molecular weights of 85.42±1.16 and 90±2.05 kDa respectively.
During estrus the protein with molecular weight range of 65-70 kilodalton were absent. Although proteins with mean molecular weight in the range were 65-70 present during proestrus.
Uterine Fluid Proteins During Luteal Phase of Estrus Cycle
The mean molecular weights of proteins during the luteal phase were 14.12±0.69, 30.37±1.17, 45.87±1.42, 67±1.57, 75.12±1.36, 77.62±3.12, and 160.75±3.97 kDa. High molecular weight (range 160-165 kDa) protein bands were absent during estrous but were present during luteal phase of estrus cycle. Low molecular weight (range between 10-15, 30-35, and 45-50 kDa) proteins were specific during the luteal phase and absent during the estrus phase. Proteins with molecular weight of 75 and 80 kDa showed two bands on SDS-PAGE.
Protein bands with mean molecular weights between 91-159 kDa were absent during luteal phase. Also proteins with molecular weights of range 15-20, 30-45, 60-65 and 205-210 kDa were not found during the luteal phase of the estrous cycle.
Early Pregnancy
The mean molecular weights of protein bands found during early pregnancy were 18.57±0.92, 30.14±1.47, 41.71±1.12, 66±1.51, and 76±2.5 and 89.57±2.83 kDa. Higher molecular weight proteins (above 91 kDa) were not found during early pregnancy. Also proteins with molecular weight in range of 10-15, 35-40, and 45-65 kDa were not found in any of the pregnant samples although they were present in luteal phase samples. Protein bands with molecular weight of 15-20 and 40-45 kDa were only found during early pregnancy.
Comparison of Proteins During Different Reproductive Stages
During the follicular phase of estrus cycle proteins with molecular weight of 10-35, 40-50 kDa range were absent. During proestrus proteins with molecular weight range between 75-80 kDa were absent whereas they were present during all other reproductive stages (estrus, luteal phase, early pregnancy). Proteins with molecular weights ranging between 205-210 kDa were present during estrus phase only. The frequency of these bands was 12.9%. Proteins with molecular weight between in the range 160-165 kDa were present both during proestrus and luteal phase (non pregnant sample) whereas these were absent in early pregnant samples. These bands represented the highest frequency (18.2%) in all bands evaluated. Proteins with molecular weight in the range of 65-70 and 75-80 kDa were absent during estrus and proestrus respectively. Protein with molecular weight in the range between 60-65 kDa were absent during luteal phase and early pregnancy. Protein bands with molecular weight in the range between 7-10 kDa were not found in any of the reproductive phase (proestrus, estrus, luteal phase and early pregnancy) studied. Also proteins bands with MWs between 90 and 160 kDa were not found in any of the samples. Similarly protein beyond the molecular weight of 210 kDa could not be evaluated. A comparison of similar protein bands during different reproductive stages by ANOVA revealed non-significant differences.
Discussion
In the present study protein bands with mean molecular weight in the range of 35-40 kDa were found during the follicular (pro-estrus and estrus) phases only, when the plasma concentration of estrogens is high. This may be a reflection of the effects of estrogens on the synthesis of this protein fraction. Presence of these proteins in high values in the follicular phase of the cycle, agrees with the report of the presence of estrogen dependent uterine serpin expression in the bovine endometrial glandular epithelium and lumen (Ulbrich et al., 2009). Also the present findings are similar to those recorded by Alavi-Shousthari et al. (2008) who recorded the presence of 38 kDa proteins during proestrus and estrus in the uterus of cattle.
In the present study protein bands with MWs between 160-165 kDa were found during proestrus and luteal phase and similar finding was recorded in a previous study on cattle (Alavi-Shoushatri et al., 2008). High molecular weight proteins (205-210 kDa) were found in the present study on buffalo uterine luminal fluids during estrus. Alavi-Shoushatri et al. (2008) found in their study on uterine fluid from cattle that 210 kDa proteins are present only in estrus samples.
In the present study proteins with MWs of 85-90 kDa were observed during follicular phase (proestrus and estrus) and early pregnancy but absent during luteal phase. During the pre-implantation period (Day 13) 29 proteins were more abundant in bovine uterus including 13 unique proteins on Day 13 compared with Day 7 (Mullen et al., 2012a).
Protein bands with mean molecular weight of 14.12 kDa were observed only in luteal phase, when ovulation, fertilization and embryo transportation to the uterus occur in the bovine and the concentration of estrogens in the plasma is very low and progesterone concentrations are increasing. Mullen et al. (2012b) had previously recorded that during the luteal phase the retinol binding protein (RBP-4) mRNA and protein concentrations are altered and increase sequentially from Day 7 to Day 13 in heifers with high plasma progesterone.
The observation of 18.57 kDa protein band, found only in early pregnancy suggests that its synthesis is under the influence of high blood plasma progesterone concentrations. This is almost similar with the range of retinol binding acidic proteins (19–22 kDa) that are progesterone dependant, and detected in pig and bovine uterine secretions which presumably helps in vitamin A transport to the fetus (Mullen et al., 2012b). Davoudi et al. (2015) demonstrated that mice uterine tissue remodeling was stimulated by progesterone treatment subsequent to estrogen treatment suggesting that the protein synthesis alterations are steroid dependent.
Protein bands with mean molecular weights of 30.37 and 30.14 kDa were recorded in luteal phase and early pregnancy in the present study. Similar findings have been previously recorded in bovine species (Alavi-Shoushtari et al., 2008; Mullen et al., 2012a). Observation of this protein band in met-estrus and in early pregnancy suggests that its release into the uterine lumen is regulated by the change in the steroid hormone concentrations in the blood plasma from a state of estrogen to progesterone dominance.
Protein bands with mean molecular weight of 41.71 kDa were observed only in early pregnancy in the present study, when the uterus is under the progesterone dominance, and protein bands with mean molecular weight of 45.87 kDa were observed only in met-estrus, when estrogen and progesterone concentrations are low. Skowronski, (2010) have previously shown that the endometrial expression of aquaporin 5 proteins in the pig uterus did not change significantly between Day 2-4 and 10-12 of the estrous cycle but increased on Days 14-16 and 18-20 as well as during early pregnancy.
Protein bands with mean molecular weight of 207.28 kDa were observed only in estrus, probably released under the influence of plasma estrogen concentrations. Our results are nearly similar with the study by Alavi-Shoushatri et al. (2008) who found in their study on cattle uterine fluids that 210 kDa proteins are present only in estrus.
Protein bands with mean molecular weight of 30.37 and 30.14 kDa were recorded in luteal phase and early pregnancy. Similar to our results the endometrial expression of a 29 kDa protein aquaporin 1 increased significantly in pregnant gilts on Days 14-16 and on Days 30-32 (Skowronski, 2010). Hettinger et al. (2001) also found an immuno-reactive 30 kDa protein in endometrial explants culture medium of gilts on Days 12, 15 and 18 of pregnancy. The uterine luminal proteins during follicular and luteal phase of estrus have shown differential activity in the uterine lumen. The proteins during follicular phase enhanced phagocytic activity in the uterus whereas those collected during the luteal phase suppressed phagocytic activity of PMNs in the buffalo uterus (Ramadan and Hassan, 1999).
Proteins with molecular weight range between 65-70 (with mean 69.71, 67, 66 kDa respectively for pro-estrus, luteal phase and early pregnancy respectively) kDa were observed in all the phases of the cycle expect estrus in the present study. Fluctuations of this protein fraction during the estrous cycle may be the result of hormonal changes.
Similar to a previous study in cattle (Alavi-shoushatri et al., 2008) protein bands with mean molecular weight range between 75-80 kDa were found in all reproductive phases expect pro-estrus in this study. Protein bands with molecular weights between 85-90 kDa were found in all reproductive stages expect in luteal phase in the present study. Specific uterine proteins such as ceruloplasmin, alkaline phosphatase (80-100 kDa) involved in tissue remodeling were observed during luteal phase in ewes (Soleilhavoup et al., 2016).
Absence and difference in protein bands during different reproductive stages was indicative of selective transfer of proteins from blood. Selective transfer of serum proteins to uterine fluid is conferred by the permeability properties of endometrial microvasculature and uterine epithelium that serves as a rate-limiting boundary for transfer of substance due to the presence of gap junctions at the apical region in between the cells. Moreover, the progesterone concentrations and the presence of conceptus alter the endometrial expression of many genes that regulate protein secretion into the uterine lumen (Forde, 2013).
More number of protein bands in uterine fluid in this study indicated that possibly proteins in uterine fluid should be originating by synthesis from uterine cell. Evidences indicate that uterine endometrium synthesizes and secretes varieties of proteins although in the absence of histochemical localization it is not always clear whether epithelial and or stromal cells are primarily responsible. However, it is suggested that the majority of the uterine specific secretory products are synthesized by the luminal and glandular epithelium and secreted into the luminal extracellular fluid (Forde, 2013). The differential abundance of several proteins during estrus, luteal phase and early pregnancy suggest their possible roles in sperm transport, endometrial remodeling and implantation (Soleilhavoup et al., 2016).
Conclusion
It was concluded that the proteins in the uterine luminal fluid change during different reproductive stages in buffalo with low MWs proteins being common during luteal phase and pregnancy and high MWs proteins common during follicular phase.
Acknowledgements
The authors acknowledge the help and permission provided by the Head Department of Animal Breeding and Genetics, Veterinary College, Bikaner. Also the permission and help by the Dean, Veterinary College, Bikaner is thankfully acknowledged.
Conflict of interest
The authors have no conflict of interest.
Authors Contribution
Part of MVSc research work carried out by Devender Kumar under the guidance of Prof Govind Narayan Purohit.
References






