Journal of Animal Health and Production
Research Article
Synergetic Effects of Multispecies Probiotic Supplementation on Certain Blood Parameters and Serum Biochemical Profile of Broiler Chickens
Sahar Farouk Deraz*
Department of Protein Research, Institute of Genetic Engineering and Biotechnology, City of Scientific Research and Technological Applications, Bourg Elarab, Alexandria, Egypt.
Abstract | The present study carried out to evaluate the effects of a multispecies probiotic isolates namely, Lactococcus lactis ssp. Lactis (Lact. lactis) and Lactobacillus plantarum (L. plantarum) on certain hematological parameters, white blood cells (WBCs), red blood cells (RBCs) counts and hemoglobulin (Hb) and serum biochemical contents, lipid profile, total proteins and serum glucose (PG), in broiler chicks. Two hundred and ten 1-day old Hubbard broiler chicks were randomly divided into seven experimental groups and fed a basic diet with 22.4% protein and 3160 kcal/kg. The experimental groups include control group and six treatment groups that supplemented probiotic in water at final concentration of 109cfu/mL and/or 1012cfu/mL of Lact. lactis and L. plantarum separately or in combination for period of 42 days and tested on scheduled intervals. The hematological analysis revealed a high significant effect (p<0.05) on counts of WBCs, RBCs and Hb concentration in all probiotc-treated groups from age of 14 days with a highly obvious increase at the experiment end (42 day). In addition, probiotic supplemented groups showed the lowest serum cholesterol, triglyceride, and total lipid contents as well as higher contents of blood glucose and total protein compared to the control group. Based on these results, it could be concluded that administration of Lact. Lactis and/or L. plantarum improves the physiological traits of broilers.
Keywords | Probiotic, Broiler, Serum biochemistry, Hematology, Poultry
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | March 01, 2018; Accepted | March 23, 2018; Published | March 26, 2018
*Correspondence | Sahar Farouk Deraz, Department of Protein Research, Institute of Genetic Engineering and Biotechnology, City of Scientific Research and Technological Applications, Bourg Elarab , Alexandria, Egypt; Email: sahar_deraz@hotmail.com
Citation | Deraz SF (2018). Synergetic effects of multispecies probiotic supplementation on certain blood parameters and serum biochemical profile of broiler chickens. J. Anim. Health Prod. 6(1): 27-34.
DOI | http://dx.doi.org/10.17582/journal.jahp/2018/6.1.27.34
ISSN | 2308-2801
Copyright © 2018 Deraz et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
One of the major challenges facing poultry industry in the developing countries, including Egypt, is limiting the vulnerability to potentially pathogenic microorganisms which results in growth performance reduction and disease incidence increasing. The prophylactic use of the antimicrobial compounds mainly antibiotics in poultry feeds have been improve the health status and has made intensive improvement in performance of birds through controlling the bacterial population present in the gastrointestinal tract (Fairchild et al., 2001; Hernandez et al., 2004). However, in the subsistence of low levels of antibiotic, antibiotic resistant bacterial cells are stimulated and grow which consider as a human health threat (Turnidge, 2004). Consequently, restrictions and limitation on the use of antibiotics for broilers have been imposed in many countries that resulted in condensation of the scientific efforts to develop efficient alternatives which could safely replace those antibiotics associated with human treatment. As a result, several new natural feed additives have drawn increased awareness as potential antibiotic growth promoter replacements including, additives of plant origin material (herbs, spices, and various plant extracts), organic acids, enzymes, phytogenic preparations as well as various probiotic (Hernandez et al., 2004; Giedrius et al., 2008). The use of probiotics feed additives as an antibiotic substitute in poultry nutrition is currently the focus of many investigations and have been successfully evaluated (Frizzo et al., 2010; Satık and Günal, 2017). Numerous qualifiers of probiotics have been introduced beginning from Fuller (1989) who defined probiotics as a live microbial feed supplement which beneficially affects the host by improving its intestinal microbial balance. However, Food and Agriculture Organization and World Health Organization (2001) have adopted another definition stated that, probiotics are: live microorganisms which when administered in sufficient amounts confer a health benefit on the host. Probiotics have been shown to have many benefits to the host animal but the most substantial one is that probiotic additives neither has any residues in animal production nor exerts any antibiotic resistance by consumption (Alkhalf et al., 2010). Many other positive impacts of probiotic supplementation in poultry have been well documented, including, accelerate development of normal microflora in the newly hatched chicks and protecting them against enteropathic disorders (Timmerman et al., 2005; Bansal et al., 2011), improving production performance, such as, feed intake, feed conversion efficiency and weight gain (Cruywagen et al., 1996; Cavit, 2003; Lesmeister et al., 2004; Awad et al., 2009; Alkhalf et al., 2010). Furthermore, the most important effect that it exerts body’s resistance to infectious diseases (Santos and Ferket, 2006) and help lowering of chick mortality (Dhama et al., 2008; Hatab et al., 2016).
The supplement of either pure Lactobacillus cultures or mixtures of lactobacilli to broiler diets has produced changeable effects (Olnood et al., 2015). Lactobacillus strains were capable in exerting consistent improvement in body weight gain and feed conversion ratio of broilers fed either a single strain of Lactobacillus or a mixture of lactobacilli from 1 to 42 days of age (Jin et al., 1998; Awad et al., 2009; Cao et al., 2013). However, Ashayerizadeh et al. (2011) did not detect any considerable difference in the performance of chickens fed on diets containing a mixture of Lactobacillus cultures and other bacteria, compared with the control group with non-supplemented diet. However, variation in the efficacy of probiotic on growth performance of broiler chickens could be attributed to many factors, such as age of animals, strain of microorganism, and inclusion level (Chen et al., 2006).
Most of the previous researches on probiotic exploitation in poultry focused on the use of monospecies probiotics and various strains of Lactobacillus. The present study was planned to investigate the effects of a multispecies probiotic isolates namely Lact. lactis and L. plantarum either separately and/or in combinations using different inclusion levels on some hematological and serum biochemical contents of one day to 42 days old broiler chicks.
Materials and Methods
Bacterial Strains
Probiotics and bacteriocin-producing LAB isolates namely, Lact. Lactis and L. plantarum were used for probiotic preparations (Deraz, 2017; Khalil et al., 2012). Stock cultures of both strains were stored at -80°C in MRS medium containing 25% (v/v) glycerol as a cryoprotectant. To produce fresh working cultures, strains were propagated twice in MRS at 37°C for 16-18 hr before experimental use.
Animals
Hubbard chicks of 1-day were purchased from Poultry Research Center, Faculty of Agriculture, Alexandria University. Chicks were caged in wire floor batteries under controlled environmental house. All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals, National Institutes of Health (Clark et al., 1997.
Husbandry
The husbandry was conducted at Poultry Research Center, Faculty of Agriculture, Alexandria University. Two hundred and ten broiler chicks of 1-d age were randomly divided into seven groups, 30 chicks each and kept for an experimental period of 42 days. Experimental diets were formulated to provide chicks with 22.4% protein and 3160 kcal/kg. Feed and water were provided ad libitum. Fresh water was provided on a daily basis during the experiment period to all the pens to ensure the viability of the probiotic culture. Remaining water from the previous day was discarded before adding fresh water, including that from pens receiving the probiotic in drinking water. To reach the target application rate of probiotics, expected water consumption was estimated based on the age of broilers.
Experimental Design and Probiotic Treatments
The randomly divided groups were treated as follows: The first group was provided diets and water ad libitum without any addition and considered as a control group. The remaining groups were supplemented with probiotic strains at various microbial concentrations. Groups 2 and 3 (T1 and T2) were provided with Lact. lactis 109cfu/mL and 1012cfu/mL, respectively. Groups 4 and 5 (T3 and T4) were provided with L. plantarum 109cfu/mL and 1012cfu/mL, respectively. Finally, groups 6 and 7 (T5 and T6) were provided with a combination of both probiotic strains with different concentrations. T5 received 1012cfu/mL Lact. lactis plus 109cfu/mL L. plantarum. T6 received 109cfu/mL Lact. lactis plus 1012cfu/mL L. plantarum. The intended LAB per milliliter of drinking water was either 109cfu or 1012cfu of each strain. To check for actual numbers, 10-fold dilutions of drinking water samples were plated on MRS agar plates in duplicate then incubated overnight at 37°C. The actual measured probiotic concentration in water samples was determined and was consistently at the required concentration throughout the experimental period.
Blood Sampling
Blood samples (2-mL) were withdrawn from 3 selected
chicks of each treatment via brachial venipuncture, collected in plastic tubes with anticoagulants, placed inside an ice box and then transferred to the laboratory. The blood samples were then divided into two portions, one for hematological analysis and one for serum analysis.
Hematological Measurements
Within 1 h after blood collection, the hematological parameters viz., white blood cells (WBCs), red blood cells (RBCs) counts and hemoglobulin (Hb) were determined using automatic blood cell analyzer (Hemavet 950FS).
Serum Biochemistry
Blood samples were collected into dry clean centrifuge tubes containing drops of heparin, then centrifuged for 15 min at 3500 rpm to obtain serum, and stored at -20°C for later analysis.
Total cholesterol (TCh), total lipid and total triglycerides (TG) concentration (mg/dL) were determined according to Bogin and Keller, (1987), Zollner and Kirsch, (1962) and Fossati and Prencipe, (1982), respectively. Total protein concentration (g/dL) was measured by the Biuret method as described by Armstrong and Carr, (1964). Serum glucose (PG) concentration (mg/dL) was estimated using the method of Trinder, (1969).
Statistical Analysis
Data were analyzed by analysis of variance using the general linear model procedure (Statistical Analysis System (SAS), 2001). Differences among means were determined using Duncan test (Duncan, 1955).
Results and Discussion
Effect of Probiotic Supplementation on Hematological Parameters
Administration of probiotic preparations containing Lact.
lactis and/or L. plantarum at concentrations of either 109cfu/mL or 1012cfu/mL to chickens caused a significant increase in total white blood cells (WBCs), red blood cells (RBCs) counts and hemoglobulin (Hb) concentration in blood plasma compared to control group (Tables 1, 2 and 3).
The counts of total WBCs were significantly increased (p<0.05) in almost all treated groups in comparison to control from 14 and up to 42 day of age (Table 1). The more prominent increase (p<0.05) in group T3 containing L. plantarum at level of 1012cfu/mL at 42 days with WBCs counts of 242.57 compared to control with a value of 158.53 (Table 1). Furthermore, probiotic-treated groups of 14 days age showed almost two fold higher RBCs counts ranged from 1.84 to 1.92 compared to control group with RBCs count of 1.07 (Table 2). However, in general the mean values of RBCs count were almost similar in all probiotic treated groups with values ranged from 2.30 to 2.37 compared to control group with value of 1.89 (Table 2). Moreover, broilers co-received both types of probiotic strains separately or in combination had a significant increase (p<0.05) in Hb concentrations (Table 3). The Hb concentrations (g/dL) were obviously increased in almost all treated groups in comparison to control along the rearing period. The more prominent increases were in groups T6 and T5 with mean values of 13.87 and 12.70 (g/dL), respectively, compared to control 9.89 (g/dL) at 42 days (Table 3).
These findings are in agreement with several previous studies. Paryad and Mahmoudi, (2008) observed that the probiotic supplementation highly increased WBCs count in broiler chicks fed different levels of probiotics than those fed diets without probiotics. Also, Cetin et al. (2005) reported that the probiotic supplementation caused statistically significant increase in the erythrocyte count, hemoglobin concentration and hematocrit values of Turkeys. Strompfova et al. (2006) and Abdollahi et al. (2003) reported that supplementation of broiler diets with probiotics strain E. faecium and B. subtilis resulted in a significant increase in the concentrations of red blood cell count, hemoglobin, hematocrit values and leukocyte numbers. In contrast, the findings disagree with the study done by Dimcho et al. (2005) who found that the probiotic supplementation did not affect the blood constituents comprising, haemoglobin concentrations. The obtained enhanced effects would be explained as dietary probiotic supplementation positively influencing blood-cell forming processes as a result of bestead iron salt absorption from the small intestine and better vitamins B production (Kander, 2004). Over and above, increased blood WBCs count could be linked to more production of immune cells (Gaggıa et al., 2010) which function in defending the biological system against different diseases (LaFleur and LaFleur, 2008).
Effect of Probiotic Supplementation on Certain Serum Constituents
Lipid Profile: The impact of supplementation by Lact. lactis and/or L. plantarum at concentrations of either 109cfu/mL or 1012cfu/mL to drinking water of chickens during their entire rearing period on serum lipid profiles are presented in Tables 4, 5 and 6.
Supplementation with probiotics resulted in numerically high improvements in total cholesterol concentrations (TCh), total lipid and total triglycerides (TG) compared to control group. Moreover, mean values of different lipid profile contents of broilers after 42 days of experimental period were not significantly affected by types or doses of both probiotic strains tested. Although, the statisticallyinsignificant decrease in TCh, total lipid and TG, chicken g-
Table 1: Values (X± SE)* of white blood cell count (×103/µL) of broiler chickens given Lact. lactis or/ and L. plantarum alone (Treatments 1, 2, 3 and 4) or in combination (Treatments 5 and 6).
| Periods |
Treatments* |
P-value | ||||||
| Control | T1 | T2 | T3 | T4 | T5 | T6 | ||
| 14 Days |
134.90 ±3.52b |
166.83 ±2.77a |
170.40 ±2.25a |
172.00 ±3.40a |
172.93 ±2.50a |
168.77 ±3.56a |
168.90 ±0.56a |
0.001 |
| 28 Days |
150.83 ±3.43c |
181.37 ±3.60b |
182.53 ±1.95b |
187.50 ±3.46ab |
194.90 ±2.60a |
187.80±3.99ab |
195.53 ±4.08a |
0.001 |
| 42 Days |
158.53 ±2.39b |
207.60 ±5.38a |
219.87 ±1.71a |
242.57±24.8a |
213.30 ±7.51a |
213.80 ±6.79a |
219.77 ±2.63a |
0.003 |
| Mean |
148.09 ±3.82b |
185.27 ±6.30a |
190.93 ±7.51a |
200.69 ±13.0a |
193.71 ±6.31a |
190.12 ±6.99a |
194.73 ±7.48a |
0.001 |
abc Means in a row with different superscripts are significantly different (P ˂ 0.05).
*The chickens in probiotic treated groups were fed either with Lact. lactis 109cfu/mL (T1), Lact. lactis 1012cfu/mL (T2), L. plantarum 109cfu/mL (T3), L. plantarum 1012cfu/mL (T4), Lact. lactis 1012cfu/mL plus L. plantarum 109cfu/mL (T5), Lact. lactis 109cfu/mL plus a L. plantarum 1012cfu/mL (T6).
Table 2: Values (X± SE)* of red blood cell count (× 106/µL) of broiler chickens given Lact. lactis or/and L. plantarum alone (Treatments 1, 2, 3 and 4) or in combination (Treatments 5 and 6).
| Periods |
Treatments* |
P- value | ||||||
| Control | T1 | T2 | T3 | T4 | T5 | T6 | ||
| 14 Days |
1.07 ±0.21b |
1.88 ±0.01a |
1.86 ±0.03a |
1.84 ±0.03a |
1.88 ±0.06a |
1.92 ±0.02a |
1.85 ±0.05a |
0.001 |
| 28 Days |
2.20 ±0.08b |
2.43 ±0.03a |
2.48 ±0.03a |
2.45 ±0.02a |
2.44 ±0.06a |
2.52 ±0.04a |
2.48 ±0.06a |
0.013 |
| 42 Days |
2.41 ±0.04c |
2.58 ±0.02b |
2.67 ±0.05ab |
2.64 ±0.03ab |
2.79 ±0.11a |
2.67 ±0.02ab |
2.65 ±0.05ab |
0.009 |
| Mean | 1.89 ±0.22 | 2.30 ±0.11 | 2.33 ±0.12 | 2.31 ±0.12 | 2.37 ±0.14 | 2.37 ±0.12 | 2.33 ±0.12 |
0.212 |
abc Means in a row with different superscripts are significantly different (P ˂ 0.05).
*The chickens in probiotic treated groups were fed either with Lact. lactis 109cfu/mL (T1), Lact. lactis 1012cfu/mL (T2), L. plantarum 109cfu/mL (T3), L. plantarum 1012cfu/mL (T4), Lact. lactis 1012cfu/mL plus L. plantarum 109cfu/mL (T5), Lact. lactis 109cfu/mL plus a L. plantarum 1012cfu/mL (T6).
Table 3: Values (X± SE)* of hemoglobin (g/dL) of broiler chickens given Lact. lactis or/and L. plantarum alone (Treatments 1, 2, 3 and 4) or in combination (Treatments 5 and 6).
| Periods |
Treatments* |
P- value | ||||||
| C | T1 | T2 | T3 | T4 | T5 | T6 | ||
| 14 Days |
8.03 ±0.19c |
9.23 ±0.38ab |
9.50 ±0.36a |
9.10 ±0.23ab |
8.84 ±0.23abc |
8.81 ±0.13abc |
8.39 ±0.46bc |
0.059 |
| 28 Days |
9.28 ±0.20b |
10.23 ±0.13a |
10.55 ±0.08a |
10.60 ±0.31a |
10.06 ±0.07a |
10.60 ±0.26a |
10.29 ±0.18a |
0.004 |
| 42 Days |
9.89 ±0.06c |
11.77 ±0.43abc |
11.53 ±0.20bc |
12.00 ±0.21abc |
11.87 ±0.26abc |
12.70 ±0.59ab |
13.87 ±1.58a |
0.035 |
| Mean | 9.07 ±0.28 | 10.41 ±0.41 | 10.53 ±0.32 | 10.57 ±0.44 | 10.25 ±0.45 | 10.70 ±0.59 | 10.85 ±0.93 |
0.259 |
abc Means in a row with different superscripts are significantly different (P ˂ 0.05).
*The chickens in probiotic treated groups were fed either with Lact. lactis 109cfu/mL (T1), Lact. lactis 1012cfu/mL (T2), L. plantarum 109cfu/mL (T3), L. plantarum 1012cfu/mL (T4), Lact. lactis 1012cfu/mL plus L. plantarum 109cfu/mL (T5), Lact. lactis 109cfu/mL plus a L. plantarum 1012cfu/mL (T6).
roups fed with various levels and types of probiotic showed the lowest mean values of TCh content of 99.8 ±12.3 and 83.52 ±11.7 mg/dL compared to the control birds 112.0 ±13.2 mg/dL (Table 4). Also, the lowest mean values of TG content (22.05 ±3.66 mg/dL) compared to the control birds (33.66 ±3.78 mg/dL) (Table 5) were in probiotic supplemented groups T2 and T5. Similar observances found with total lipid (Table 6). Among the 6 doses of applied probiotic treatments, T1 group and T5 group reduced the mean total lipid content to 385.8 ±21.0 mg/dL and 366.33 ±17.6 mg/dL compared to control with 431.1 ±17.1 mg/dL, respectively (Table 6). Our observations are in agreement with numbers of previous literature (Mansoub, 2010; Amer and Khan, 2012). White Leghorn layers supplemented with a commercial probiotic product (Protexin probiotic) showed lower serum cholesterol level from 176.5 to 114.3 mg/dL (Mohan et al., 1995). In another study by Mohan et al., 1996) who reported that broilers that provided with 75, 100, and 125 mg probiotic/kg diets brought down the serum cholesterol content to 93.3 mg/dL
Table 4: Values (X± SE)* of total cholesterol (mg/dL) of broiler chickens given Lact. lactis or/and L. plantarum alone (Treatments 1, 2, 3 and 4) or in combination (Treatments 5 and 6).
| Periods |
Treatments* |
P- Value | ||||||
| Control | T1 | T 2 | T 3 | T 4 | T 5 | T6 | ||
| 14 days | 123.8±25.1 | 103.1 ±23.5 | 97.6 ±14.1 | 96.4±18.8 | 120.3±36.3 | 92.2±21.4 | 96.4 ±25.1 | 0.954 |
| 28 Days | 99.2 ±27.2 | 117.89±17.0 | 95.9 ±23.8 | 122.8±28.9 | 103.0±36.4 | 83.4 ±18.0 | 109.1 ±15.8 | 0.980 |
| 42 Days | 113.0±23.8 | 106.7 ±24.6 | 105.8±320 | 98.9 ±33.2 | 92.4 ±13.4 | 75.08 ±28.6 | 117.8 ±28.8 | 0.996 |
| Mean | 112.0±13.2 | 109.2 ±11.2 | 99.8±12.3 | 106.0±14.4 | 105.2±15.9 | 83.52 ±11.7 | 107.8 ±12.3 | 0.995 |
*The chickens in probiotic treated groups were fed either with Lact. lactis 109cfu/mL (T1), Lact. lactis 1012cfu/mL (T2), L. plantarum 109cfu/mL (T3), L. plantarum 1012cfu/mL (T4), Lact. lactis 1012cfu/mL plus L. plantarum 109cfu/mL (T5), Lact. lactis 109cfu/mL plus a L. plantarum 1012cfu/mL (T6).
Table 5: Values (X± SE)* of total triglyceride (mg/dL) of broiler chickens given Lact. lactis or/and L. plantarum alone (Treatments 1, 2, 3 and 4) or in combination (Treatments 5 and 6).
| Periods |
Treatments* |
P- Value | |||||||
| Control | T1 | T 2 | T 3 | T 4 | T 5 | T6 | |||
| 14 Days | 26.95 ±6.44 | 31.17 ±5.87 | 21.04 ±5.07 | 35.17 ±3.68 | 34.96 ±6.15 | 39.44±6.16 | 29.17 ±6.85 | 0.412 | |
| 28 Days | 36.36 ±3.08 | 24.04 ±2.62 | 21.38 ±5.43 | 38.89±10.69 | 20.60 ±4.98 | 30.54±2.08 | 29.84 ±5.53 | 0.210 | |
| 42 Days | 37.67 ±9.28 | 16.55 ±8.45 | 23.73±10.18 | 24.18 ±5.03 | 27.47 ±2.05 | 20.50±10.27 | 24.77±12.49 | 0.762 | |
| Mean | 33.66 ±3.78 | 23.92 ±3.72 | 22.05 ±3.66 | 32.75 ±4.20 |
27.68 ±3.14 |
30.16±4.45 | 27.93 ±4.48 | 0.327 | |
*The chickens in probiotic treated groups were fed either with Lact. lactis 109cfu/mL (T1), Lact. lactis 1012cfu/mL (T2), L. plantarum 109cfu/mL (T3), L. plantarum 1012cfu/mL (T4), Lact. lactis 1012cfu/mL plus L. plantarum 109cfu/mL (T5), Lact. lactis 109cfu/mL plus a L. plantarum 1012cfu/mL (T6).
Table 6: Values (X± SE)* of total lipid (mg/dL) of broiler chickens given Lact. lactis or/and L. plantarum alone (Treatments 1, 2, 3 and 4) or in combination (Treatments 5 and 6).
| Periods |
Treatments* |
P- Value | |||||||
| Control | T1 | T 2 | T 3 | T 4 | T 5 | T6 | |||
| 14 Days | 437.6 ±1.1 | 328.5 ±28.3 | 447.8 ±17.8 | 419.4 ±35.0 | 401.9 ±53.1 | 394.8 ±27.7 | 397.7 ±17.1 | 0.151 | |
| 28 Days | 474.8 ±20.5 | 421.7 ±44.4 | 446.2 ±67.9 | 389.8 ±54.8 | 354.9 ±52.1 | 355.2 ±13.6 | 471.8 ±31.6 | 0.374 | |
| 42 Days | 380.9 ±28.9 | 407.0 ±3.1 | 401.7 ±34.4 | 439.4 ±81.5 | 482.5 ±52.6 | 350.0 ±44.8 | 467.2 ±40.2 | 0.714 | |
| Mean | 431.1 ±17.1 | 385.8 ±21.0 | 431.9 ±23.8 | 416.2 ±30.9 | 413.1 ±32.2 | 366.33 ±17.6 | 445.6 ±19.6 | 0.531 | |
*The chickens in probiotic treated groups were fed either with Lact. lactis 109cfu/mL (T1), Lact. lactis 1012cfu/mL (T2), L. plantarum 109cfu/mL (T3), L. plantarum 1012cfu/mL (T4), Lact. lactis 1012cfu/mL plus L. plantarum 109cfu/mL (T5), Lact. lactis 109cfu/mL plus a L. plantarum 1012cfu/mL (T6).
compared to the control birds (132.2 mg/dL). The mechanisms that probiotics reduce total cholesterol and triglyceride could be attributed their characteristic to deconjugate bile acids enzymatically using bile-salt hydrolase (Surono, 2003). Additional couple of mechanisms was suggested. First mechanism assumed that probiotic microorganisms inhibit hydroxy-methyl-glutaryl-coenzyme A, which is a substantial enzyme for cholesterol synthesis pathway thereby, diminish cholesterol synthesis (Fukushima and Nakano, 1995). However, Mohan et al. (1995) and (1996) suggested that the ability of probiotics may be refer to either absorption and/or synthesis of cholesterol reduction in the gastro-intestinal tract by probiotic supplementations. On the other hand, Kawahara et al., (1991) did not detect any influence of added probiotics on serum cholesterol. While Owosibo et al. (2013) found that the serum cholesterol value was significantly increased by the probiotics supplementation in broiler.
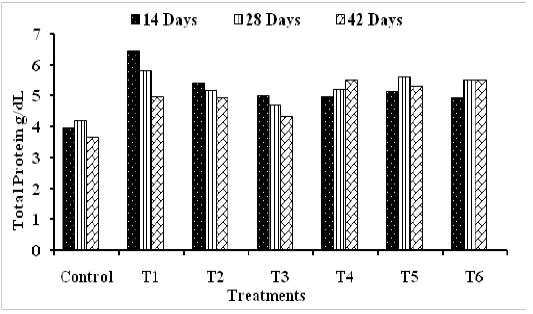
Total Protein and Serum Glucose: Broilers received both types of probiotic strains separately or in combination had an observed increase in both total protein and serum glucose (PG) concentrations (Figures 1 and 2). Probiotic-treated groups T1 and T2 showed the highest values of protein contents of 6.43 ±1.73 and 5.41 ±1.28 (g/dL) compared to control (3.96 ±0.81 g/dL) at 14 day (Figure 1). However, at 42 days aged broilers, the highest values were recorded in groups T6 followed by T4 and T5 with protein content of 5.52 ±1.20, 5.51 ±1.06 and 5.32 ±1.25 (g/dL), respectively, compared to control (3.6 5±0.99 g/dL) (Figure 1). These findings are in agreement with those of Dimcho et al. (2005) and Alkhalf et al. (2010) who found that probiotic supplementation, did not affect the total proteins concentrations of chickens. However, Agawane and Lonkar, 2004 reported improvement in protein values in broilers supplemented with Saccharomyces boulardii and attributed to increased protein synthesis as an effect of probiotic.
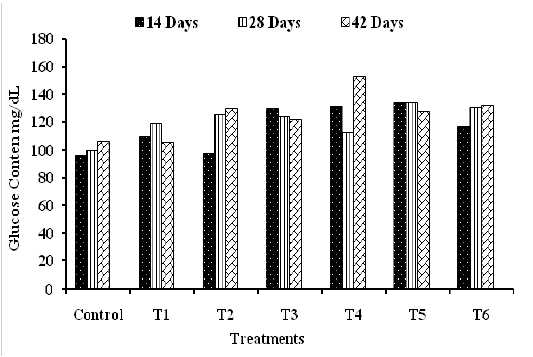
The PG concentrations were obviously increased in almost all treated groups in comparison to control along the rearing period and the more prominent increased were in groups T4, T5 and T6 with mean values of 132.0 ±8.5, 132 ±5.0 and 126.4 ±6.8 mg/dL, respectively, compared to control with mean value of 100.8 ±10.7 mg/dL (Figure 2). The obtained results were in accordance with the findings of Hussein, (2014) and Hatab et al. (2016) who detected significant increase in serum glucose concentration in broilers fed on diets supplemented with probiotics as compared to the control. The increase in the serum glucose concentration may be due to a temperate amelioration gluconeogenesis and boosted lactose absorption (Das et al., 2005). However, our results were in contrast with the findings obtained by Abd El-Baky, (2007) who recorded no changes in blood glucose level in broiler treated with probiotics. Unlikely, the results obtained by Al-Kassie et al. (2008) reported lessening in serum glucose level in groups provided with probiotics as compared to the control.
Conclusions
It could be concluded that dietary supplementation of probiotics bacteria Lact. lactis and L. plantarum to the broiler chickens could have positive effects on the hematological and serum biochemical parameters and subsequent immunity of growing broilers. This could have positive effects on broiler productive performance thereby improving the physiological and metabolic activities of the broiler body and increasing the availability of the nutrients required. It is precious to point that no any antibiotic was added up to or injected in the broilers from the first day until the end of the experiment.
Acknowledgements
The author highly acknowledged Prof. Ashraf A. Khalil and Prof. Alaa E. Elkomy for their assistance in data analysis and reviewing this paper.
conflict of interest
The authors has declared no conflict of interest.
References