Journal of Animal Health and Production
Research Article
Dose and Strain Dependent Induction of Cell Death of Human Breast Cancer Cells (Mcf-7) by Newcastle Disease Virus
Ramesh Bhanudas Jagtap1, A Raja2, M Parthiban2, A Palanisamy2
1Dept. of Animal Biotechnology, Madras Veterinary College, Tamil Nadu Veterinary and Animal Sciences University (TANUVAS), Chennai, Tamil Nadu (TN), India; 2Department of Animal Biotechnology, Madras Veterinary College, Chennai-600 007. Tamil Nadu Veterinary and Animal Sciences University, Chennai.
Abstract | Newcastle disease viruses are now considered to be safe platforms for the development of oncolytic virotherapeutics due to lack of adverse consequences in clinical trials. In this study, we aimed to assess in-vitro the tumoricidal properties of local velogenic isolate NDV/2K/17 and mesogenic isolate NDV/2K/36 of Tamil Nadu in Human Breast Cancer Cell line (MCF-7). The NDV isolates were propagated in embryonated chicken eggs and purified and concentrated by ultracentrifugation. The monolayer of MCF-7 cells was infected with three different doses (16, 8 and 4 HAU) of each virus separately and cytopathic effects were studied by staining. Cell viability/cytotoxicity in infected cells was assessed using trypan blue dye exclusion test and MTT cell viability assay. The trypan blue staining of NDV/2K/17 and NDV/2K/36 infected cell line showed up to 68% and 82 % of cell death, 72h p.i. (post infection), respectively. MTT cell viability assay at 24h, 48h, 72h p.i. by 16 HAU of the NDV/2K/17, show decreased percentage of viable cells to 83.85, 55.7 and 35.58 %, respectively as compared to uninfected MCF-7 cells. Whereas at 24h, 48h and 72h p.i. by 16 HAU of mesogenic isolate NDV/ 2K/36, percent viable cells decreased to 87.54, 54.01 and 17.78, respectively showing decreased viability percent with time post infection. Besides, both isolates were found to induce cytotoxicity in MCF-7 cells in a dose dependent manner. With increasing dose of virus, percent cell viability decreased showing minimal at 72h p.i. (35.58 % by NDV/2K/17 and 17.78% by NDV/2K/36) by higher dose of each virus (16HAU). However, with lower dose (4HAU) of each virus resulted in comparatively higher cell viability at 72h p.i. (60.93 % by NDV/2K/17 and 29.39% by NDV/2K/36) in MCF-7 cells. In conclusion, our results indicated that both isolates of NDV were cytolytic to the MCF-7 cells and cell death induced was dependent on the strain and dose of the Newcastle disease virus used.
Keywords | NDV, Velogenic, Mesogenic, Ultracentrifugation, MCF-7 cells, MTT, Cytotoxicity
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | February 04, 2017; Accepted | February 24, 2017; Published | February 28, 2017
*Correspondence | Ramesh Bhanudas Jagtap, Vidya Pratishthan’s School of Biotechnology, Vidyanagari, MIDC road, Baramati, Dist- Pune, India. Pin- 413 133; Email: docrameshjagtap@gmail.com
Citation | Jagtap RB, Raja A, Parthiban M, Palanisamy M (2017). Dose and strain dependent induction of cell death of human breast cancer cells (mcf-7) by Newcastle disease virus. J. Anim. Health Prod. 5(1): 29-34.
DOI | http://dx.doi.org/10.14737/journal.jahp/2017/5.1.29.34
ISSN (Online) | 2308-2801; ISSN (Print) | 2309-3331
Copyright © 2017 Jagtap et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Oncolytic viruses are the viruses that infect and replicate in cancer cells, selectively killing cancer cells leaving normal cells intact or largely unaffected (Reichard et al., 1992). Among the oncolytic viruses, Newcastle disease virus (NDV) is one such virus with an inherent oncolytic property (Omar et al., 2003). NDV or avian paramyxovirus 1 (APMV-1) belongs to the genus Avulavirus in the family Paramyxoviridae (Alexander, 1997; Mayo, 2002). NDV causes a highly contagious, generalised viral disease of domestic poultry and wild birds characterized by gastro-intestinal, respiratory and nervous signs. Depending on the severity of the disease, pathogenicity and clinical signs in the affected birds, NDV classified into three pathotypes, namely velogenic (highly virulent), mesogenic (moderately virulent) and lentogenic (avirulent) (Alexander, 1997; Alexander, 1989).
The selective nature of NDV makes it an ideal virotherapy agent. It is demonstrated that NDV selectively replicates in tumour cells and induces death while sparing normal cells. Due to this property, NDV has been exploited as a potential anti-cancer agent in humans (Schirrmacher et al., 2001; Lorence et al., 2003).
In India number of isolates of NDV detected and characterised from different states and geographical regions. Among the others, velogenic isolate NDV/2K/17 and mesogenic isolate NDV/2K/36 are prevalent in Tamil Nadu state. Though, number of NDV strains across globe proved their oncolytic potential in-vitro in different cancer cell lines, animal models and in clinical trials, the most of the Indian NDV isolates are yet to be tested for their oncolytic potential. So in view of developing potential alternative cancer therapeutics, investigation has been planned to assess in-vitro oncolytic properties of these two local isolates of NDV on MCF-7 cells and also to study the effect of dose or strain of NDV on the survival of the MCF-7 cells.
MATERIALS AND METHODS
Propagation Of NDV/2K/17 And NDV/2K/36 In Embryonated Chicken Eggs
The two local isolates from Tamil Nadu namely, Velogenic isolate NDV/2K/17 and mesogenic isolate NDV/2K/36 were used in the study. Both isolates were taken from Virus repository of department of Animal Biotechnology, Madras Veterinary College, Chennai. Each NDV isolate were propagated in 9-11 day old embryonated SPF chicken eggs. Briefly, each SPF chicken egg is candled and large sized, fertile, motile embryos were selected. The air cell is marked with pencil and area with no blood vessels 2-3 mm below the air cell is pointed as site of inoculation. Shell of each egg is swabbed with 70% ethanol and gently a pinpoint hole is made at point marked. Then 0.2 µl of NDV suspension aspirated in 1ml syringe is inoculated in each SPF egg. Motility of the embryos was checked every 12 hrs by candling. After 72 hrs of inoculation or after death of the embryos, about 5-6 ml of amnio-allantoic fluid (AAF) is harvested in sterile centrifuge tubes. The infected AAF was clarified by centrifugation at 5000 rpm for 30 minutes at 40C, supernatant is pipetted out in fresh sterile tubes and the presence of virus was confirmed by the haemagglutination test (Alexander, 1988).
Purification And Concentration Of NDV
For each virus about 150 ml of AAF from infected embryos is harvested in sterile tubes. Initial clarification of AAF was carried out by centrifugation at 5000 rpm for 30 minutes at 40C (Kalid et al., 2010), supernatant is pipette out in 50 ml tubes used for ultracentrifugation. Virus was further concentrated by ultracentrifugation of virus at 32000 rpm for 3 hrs. The pellet obtained was dissolved in sterile phosphate buffer saline (PBS). The virus was further purified by ultracentrifugation at 20000 rpm for 3 hrs using 30 – 60 % sucrose gradient. The visible band of virus was collected at the interphase of 40% -50% sucrose concentration and further virus was pelleted by ultracentrifugation at 32,000 rpm for 2 hours. Finally, stock of virus was prepared by dissolving virus pellet in minimal quantity of sterile 1X PBS and final dilutions were made in DMEM without serum just before infection.
Culture And Maintenance Of Cancer Cell Line
The Human breast cancer cell line (MCF-7) was procured from National Center for Cell Science (NCCS), Pune. The MCF-7 cells were cultured in growth medium; Dulbecco’s Modified Eagle media (Sigma) containing 10% foetal bovine serum (FBS) (Gibco, USA) and 1X Antibiotic Antimycotic solution (SIGMA) in a humidified 5% CO2 incubator at 370C (Galaxy 170 S, New Brunswick, Eppendorf). The maintenance medium (DMEM containing 2% FBS) used for maintaining the culture and during infection of cultures with NDV. The cells were subcultured after they had achieved 80-90% confluency which can be observed under inverted microscope (Euromex, Holland).
Infection Of Cell Line And Trypan Blue Dye Exclusion Test
After attainment of 80-90% confluency in 25 cm2 tissue culture flask, spent medium was pipetted out from the flask. The cells were incubated with 100 µl of each virus separately for 45 min. The maintenance medium was added and cells were incubated at 370C in 5% CO2 incubator. Trypan blue, 0.4% w/v (Gibco, USA), was used for viability testing. Cell viability was assessed using trypan blue exclusion test. At 72h of infection, cells were trypsinized, harvested in growth medium and washed with sterile 1XPBS (pH 7.4). The 20 µl of single cell suspension and 20 µl of trypan blue stain mixed and loaded on Neubauer’s chamber slide. The cells stained blue counted as dead and the cells without stain counted as live. The percent dead cells were determined as mentioned below:
Cells/mL = Average number of cells counted in 64 squares x 104 x dilution factor
The dead cells took blue stain and unstained cells were live cells. The live and dead cells counted separately. The NDV/2K/17 and NDV/2K/36 showed 68 and 82% cell death at 72h p.i.
H And E, And Giemsa’s Staining
The cells were grown on cover glasses in the test tubes until about 80% monolayer formed. Then cells were infected with velogenic NDV/2K/17 and mesogenic isolate ND
V/2K/36, separately. After 48h of infection, cells grown on were washed with PBS to remove traces of serum. Then cells were fixed with absolute alcohol on the glass slides and washed with tap water for 10 min. Cells incubated with hematoxylin for 1-5 min., washing with tap water repeated and stained with eosin for 1 min. Final washing was given with distilled water with few quick changes of water, air dried and mounted. Similarly, for Giemsa’s staining, cells were fixed with absolute alcohol and stained with Giemsa’s stain, dried and mounted. The cytopathic effects in NDV infected cells were observed under inverted microscope.
MTT Assay
Cell viability was assessed by MTT, (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, a yellow tetrazole). The MCF-7 cells were cultured in 96 well tissue culture plate until the 80-90% monolayer formed. The spent medium was removed by pipetting out. At 24h, 48h, 72h post infection with NDV, a volume of 15 µl MTT reagent (5 mg/ml) was added to each well and further incubated for 3 hours in a humidified 5% CO2 incubator at 370 C. After the incubation time, the medium and MTT were removed and a volume of 200 µl of DMSO was added to each well. Absorbance at 570 nm of the mixture was detected using a microplate reader (Biotek instruments).
Statistical Analysis
One-way ANOVA test was applied to compare means of treatment groups using SPSS statistics 17.0. The means bearing different superscripts differ significantly along row for each virus at 95% confidence level (p<0.05).
RESULTS AND DISCUSSION
Virus Propagation And Purification
About 200 ml of infected AAF for each isolate were collected and clarified. The clarified AAF was ultracentrifuged to pellet the virus. Virus pellet dissolved in 1 ml of sterile 1X phosphate buffered saline (PBS) and purified by ultracentrifugation of virus by using sucrose gradient. The purified virus was dissolved in 5ml of Minimum Essential Medium (MEM), incubated with 1x antibiotic antimycotic solution for 1h at room temperature and used for infection of cultures.
Haemagglutination Test
The HA titre of both velogenic isolate NDV/2K/17 and NDV/2K/36 noted was 32 Haemagglutination Unit (HAU). Both viruses diluted and three different doses of viruses 16 and 8 and 4HAU were used in the study.
Culture And Maintenance Of MCF-7 Cell Line
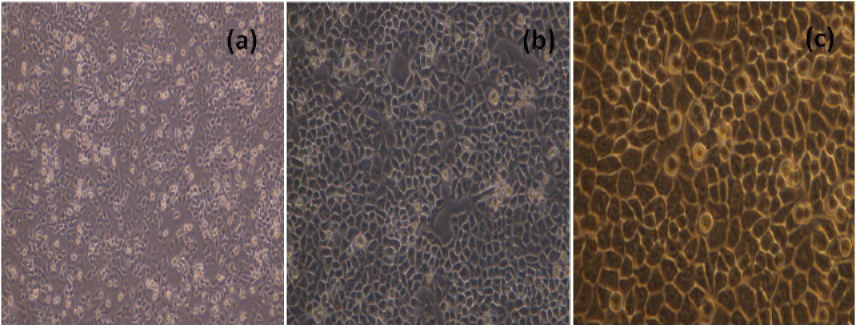
The MCF-7 cells, cultured in 25 cm2 flask were sub-cultured after every two days with split ratio of 1:3. The cultured MCF-7 cells 24h and 48h and 72h after passage were shown in Figure 1.
NDV Cytopathic Effects in MCF-7 Cell Line
The morphological changes in NDV infected cells 24h, 48h and 72h p.i were shown in Figure 2.
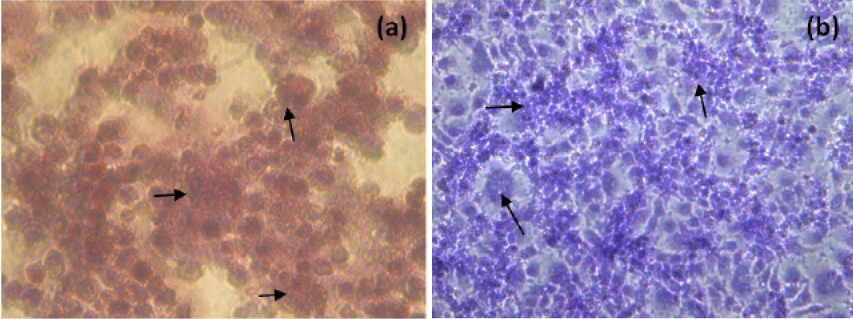
The NDV infected H and E and Giemsa stained MCF-7 cells at 48h p.i. were showed in Figure 3. NDV infection resulted in rounding of cells, fusion of cell cytoplasm and syncytia formation. Further detachment of cell monolayer was observed.
Cell viability Study By MTT Assay
The percent cell viability was assessed using MTT at 24h, 48h and 72h p.i. by NDV/2K/17 and NDV/2K/36 (Table 1). It was found that at 24h, 48h, 72h after infection by 16 HAU of the NDV/2K/17, percent viable cells decreased to 83.85, 55.7 and 35.58%, respectively as compared to uninfected MCF-7 cells (5.1% at 72h). Whereas at 24h,

Figure 2: Unstained, MCF-7 cells infected with NDV (200X), 24h (a), 48h(b) and 72h (c) p.i. Arrows indicate, rounding of cells and detachment of monolayer
Figure 3: H and E (a) and Giemsa (b) staining of NDV infected MCF-7 cells (400X, Inverted microscope). Arrows indicate, rounding of cells, fusion of cell cytoplasm and syncytia formation
48h and 72h p.i. by 16 HAU of mesogenic isolate NDV/2K/36, percent viable cells decreased to 87.54, 54.01 and 17.78 (Table 1), respectively compared to uninfected cells (4.5% at 72h). The velogenic and mesogenic strains of NDV possess polybasic cleavage site in F protein (Zamarin and Palese, 2012). Thus both strains may possess fusogenic properties leading to formation of syncytia in-vitro in cancer cell lines and causes death of cancer cells. However, other factors could also play important role in final outcome of the lytic effect of velogenic and mesogenic strains in cancer cell lines. Differences if any, among two strains in fusogenicity, replication in MCF-7 cell line need to be investigated further and which could be the reason why mesogenic NDV showed more cell death in HeLa cell line. Multiple studies confirmed role of apoptosis in NDV mediated oncolysis (Ravindra et al., 2009a; Zulkifli et al., 2009; Molouki et al., 2010; Ali et al., 2011; Fabian et al, 2001; Szeberenyi, 2003; Elankumaran et al., 2006; Fabian et al., 2007; Bian, 2011). Further, NDV causes cell death in cancer cell line by both intrinsic and extrinsic apoptotic pathways (Elankumaran et al., 2006). However, there are several other mechanisms also reported in NDV-induced oncolysis (Ravindra et al., 2009b; Elankumaran et al., 2006; Fabian et al., 2007; Bian et al., 2011). It is difficult to delineate which mechanism is involved in causing oncolysis. Differences in the cell lines, viral strains, and detection assays used could possibly the key factors in this regard (Zamarin and Palese, 2012). Moreover, different levels of activation of apoptosis pathways were noted among the different cell lines and viral strains tested (Elankumaran et al., 2006).
Besides, in this study both isolates were found to induce cytotoxicity in MCF-7 cells in a dose dependent manner. The virus titre of 8 and 4 HAU of NDV/2K/17 at 72h p.i. resulted in significant (p<0.05) decrease in cell viability percentage to 45.65 and 60.93%, respectively and 8 and 4 HAU virus titre of NDV/2K/36, 72h p.i. resulted
Table 1: Percent viable MCF-7 cells after infection with NDV analysed by MTT assay
|
Dose of the Virus |
|||||||||
|
NDV/2K/17 |
NDV/2K/36 |
||||||||
|
Hours post infection |
4HAU |
8HAU |
16HAU |
4HAU |
8HAU |
16HAU |
|||
|
Cell Viability (%) |
24h |
94.32a ±4.22 |
89.5b ±3.17 |
83.85c ±3.24 |
93.86a ±4.51 |
91.35a ±3.02 |
87.54b ±3.66 |
||
|
48h |
73.56a ±3.6 |
68.5b ±2.78 |
55.7c ±4.05 |
76.75a ±2.89 |
73.27a ±4.22 |
54.01b ±4.03 |
|||
|
72h |
60.93a ±3.51 |
45.65b ±2.9 |
35.58c ±4.2 |
29.39a ±3.2 |
23.87b ±3.89 |
17.78c ±2.53 |
|||
Means bearing different superscripts (a,b,c) differ significantly along row for each virus at p<0.05
in 23.87 and 29.39% viable cells, respectively compared to uninfected cells (Table 1). However, Othman et al. (2010), by MTT assay showed that virus titre needed for 50% killing of MCF-7 cells was 2 HAU for NDV AF2240 Strain. Further, after 24, 48h and 72h p.i. results in 24±4.08, 50 and 81±3.89% of apoptotic cell death in MCF-7 cells. Like NDV there are several other oncolytic viruses (Adenovirus, reovirus, coxasackievirus, Herpes Simplex virus 1, retrovirus, pox virus, vesicular stomatitis virus, picorna virus, measles virus, influenza virus, etc. Parato et al. (2005) which are being tested against various cancers. Besides use of oncolytic viruses to target and kill cancer cells, virus-modified tumour cell vaccines, and virus infected/ sensitised immune cells are used as an approach to cure cancer. In this scenario, among the others, Chen et al. (2016), explored therapeutic effect of combination of chimeric antigen receptor (CAR)-modified immune cells (NK-92) and oncolytic herpes simplex virus (oHSV) in MCF-7 cell line. It was found that oHSV-1 alone was capable of causing lysis of cells. However, a higher cytolytic effect of EGFR-CAR NK-92 cells was observed when combined with oHSV-1 in MCF-7 cells.
Like oncolytic viruses there are several other agents which are evaluated for anticancer effects in cancer cell lines. Among these, Selim and Hendi (2013) reported the toxic responses of gold nanoparticles to human breast epithelial MCF-7 cells. It was demonstrated that exposure of gold nanoparticles to MCF-7 cells cause cytotoxicity and the MTT assays revealed that the gold nanoparticles exert significant cytotoxicity to MCF-7 cells in dose-dependent fashion in the concentration range of 25-200 μg/ml.
CONCLUSIONS
Both isolates of NDV, induces cell death in MCF-7 cells. With increasing time after infection the percent viable cells were decreased in case of both viruses. The cell death induced by both isolates was dependent on the strain or pathotype of the virus used. Mesogenic isolate NDV/2K/36 has significantly (p<0.05) greater cytolytic potential showing lower (17.78%) cell viability at 72h p.i. than velogenic NDV/2K/36 at same time point (35.58%). Further, the induced cytotoxicity depends on the dose of the virus, infection with either virus, cell viability decreased significantly (p<0.05) with increase in dose of virus.
ACKNOWLEDGEMENT
Authors are thankful to Dept. of Animal Biotechnology, Madras Veterinary college, Tamil Nadu Veterinary and Animal Sciences University, Chennai-51, for the provision of necessary facilities and funds to carry out research work. The part of the work was carried out under ICAR-Niche scheme on avian viruses.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR’S CONTRIBUTION
The experiment was designed and performed by Ramesh B. Jagtap during his Ph. D., programme at Dept. of Animal Biotechnology, Madras Veterinary College, Chennai. A. Raja, M. Parthiban, A. Palanisamy provided necessary laboratory facilities, consumables and administrative support for the work.
REFERENCES