Journal of Animal Health and Production
Research Article
Occurrence of Anthrax Spores in Small Ruminants Hair/Wool in District Tharparkar, Sindh
Maryam Rajput1, Asghar Ali Kamboh1*, Parkash Dewani2, Aslam Parvez Umrani2, Rahmatullah Rind1
1Department of Veterinary Microbiology, Faculty of Animal Husbandry and Veterinary Sciences, Sindh Agriculture University, Tandojam 70060, Pakistan; 2Directorate of Veterinary Research and Diagnosis, Central Veterinary Diagnostic Laboratory (CVDL) Tandojam, Sindh, Pakistan.
Abstract | Bacillus anthracis (B. anthracis) is a spore-forming bacteria that causes anthrax in animals, including small ruminants. The present study, for the first time investigated the B. anthracis spores contamination in small ruminant’s wool/hair in district Tharparkar, Pakistan. Randomly selected sheep and goats (n=40 each) were used for sample collection from different body parts i.e., abdomen, neck + face, fore limbs and hind limbs. Overall results showed that 28% samples were contaminated with anthrax spores. A moderately high (P > 0.05) incidence of anthrax spores was observed in samples collected from abdomen (35%), followed by neck + face (30%), fore limbs (25%) and hind limbs (20%) respectively. Sheep wool (30%) has a higher contamination level (P < 0.05) than goat hair (25%). In sheep, the highest prevalence was observed in 2 years of age group i.e., 4/8 (50%), whereas, 3/8 (37.5%), 3/12 (25%) and 2/12 (16.6%) samples were positive in age groups of 2 ½, 3 and 4 years respectively. However, in goats the prevalence was recorded as 6/12 (50%), 3/12 (25%) and 1/8 (12.5%) in age groups of 2, 2 ½ and 3 years respectively, whereas, anthrax spores were not found in goats of 4 years age-group. These results indicated that wool/hair of small ruminants harbor B. anthracis spores. Moreover, sheep wool harbored relatively higher spore numbers as compared to goat hair.
Keywords | Anthrax Spore, Small ruminant, Tharparkar, Hair, Wool.
Editor | Sanjay Kumar Singh, Animal Gynecology Lab, Animal Reproduction Division, Indian Veterinary Research Institute, Izatnagar, Bareilly (UP), India.
Received | December 17, 2016; Accepted | January 20, 2017; Published | January 25, 2017
*Correspondence | Asghar Ali Kamboh, Department of Veterinary Microbiology, Faculty of Animal Husbandry and Veterinary Sciences, Sindh Agriculture University, Tandojam 70060, Pakistan; Email: drasgharkamboh@yahoo.com
Citation | Rajput M, Kamboh AA, Dewani P, Umrani AP, Rind R (2017). Occurrence of Anthrax spores in small ruminants hair/wool in district Tharparkar, Sindh. J. Anim. Health Prod. 5(1): 5-9.
DOI | http://dx.doi.org/10.14737/journal.jahp/2017/5.1.5.9
ISSN | 2308–2801
Copyright © 2017 Rajput et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Small ruminants (goat and sheep) are common livestock of rural community all over the globe. Sometimes, goat is known as “Poor Man’s Cow”. At present, Pakistan supports 56.7 million heads of goats, consisting of about 25 well recognized breeds found in different regions of the country (Pirzada et al., 2016). Likewise, there was a 28.8 million heads of sheep that produced 43.6 thousand tons of wool. Usually small flocks of sheep and goats are raised together in plains and sub hilly areas of Pakistan (GoP, 2013-14; Panicker et al., 2015).
Under natural conditions, sheep and goat are highly susceptible to a number of bacterial pathogens like those cause blackleg, foot rot, caprine pleuropneumonia, contagious bovine pleuropneumonia, chlamydiosis, anthrax etc. (Peter et al., 2015). Anthrax is a primarily disease of herbivores, although all mammals, including humans, and a few avian species can contact it. The disease has worldwide distribution and is a zoonosis. Mortality can be very high, especially in herbivores. The disease is caused by Bacillus anthracis, which is a spore-forming, Gram positive, rod-shaped, non-motile and obligate pathogen of genus Bacillus (Hendricks et al., 2014). Sporulation only occurs when the vegetative form is exposed to the atmosphere and conditions are unfavorable for the continued multiplication of the vegetative form. Anthrax spores are resistant to heat and chemical disinfectants (Coffin et al., 2015; Kracalik et al., 2013). To prevent environment contamination, it is usual not to perform a necropsy on the carcass of suspected or confirmed cases. If the carcass is opened either by necropsy or scavengers, the vegetative form of B. anthracis is released from the acidic environment of decay and produce spores that create foci of contamination (OIE, 2004). Open wounds / skin cuts of animals may get anthrax spores from surroundings including soil, feeding troughs, farm utensils, feed, water etc. (Peter et al., 2015; WHO, 2008).
B. anthracis is found in areas where anthrax spores are located. Anthrax is transmitted to livestock animals through ingestion of spores via contaminated feed or water. The frequency of infection may rise as a result of drought or malnutrition. Routes of transmission by biting flies or contact with contaminated feces have also been reported (OIE, 2004; Zaidan et al., 2015). Soil is being contaminated with the spores from the carcasses of infected animals (Dragon et al., 2001). Another source of spores in soil may be contaminated effluent from industrial plants working with animals products such as leather, tanneries etc. The ability of these spores to remain viable for years in animal’s products, soil and industrial environment is an important factor in the epidemiology of anthrax and explain the predominant occurrence of the disease in herbivores (OIE, 2004).
Previous studies have investigated the prevalence of B. anthracis spores in soil of various geographical regions. To the best of our knowledge, no study is available that have explored the occurrence of B. anthracis spores on the skin of livestock animals. We hypothesized that, skin remained in direct contact with the soil, so it may get spores from soil (if it harbored). Therefore, the present study was planned to explore the occurance of B. anthracis spores on the skin of small ruminants in Tharparkar, Sindh, Pakistan.
Materials and Methods
Study Area and Safety Precautions
The study was carried out in Tharparkar district. This district has an overall area of 19638 km that lies to the southeast of Sindh province, Pakistan. This is one of the most populated desert areas in the world. The Tharpark district faces severe drought usually after every few years. Rainfall is uneven with an annual average of 50-300 mm (Rana and Naim, 2014). The people of Tharparkar rely only on rain-dependent agriculture and animal husbandry.
Anthrax is a disease listed in the World Organization for Animal Health (OIE) Terrestrial Animal Health Code (the Terrestrial Code). The Terrestrial Code has set a standard to carry out research on anthrax with a minimum risk to the health of the staff (biosafety) and the environment (biocontainment). These standard measures were adopted during sample collection and analysis in the labaratory. Personal protective equipment such as long-sleeved lab coats or gowns, closed-toe footwear, disposable gloves, masks, safety glasses, face shields, and oro-nasal respirators, as appropriate, were worn during sample collection and also in the laboratory, and removed when leaving the laboratory.
Collection of Samples
Samples were collected from randomly selected 20 villages of district Tharparkar. From each village two sheep and goats were used for wool and hair collection using an electric sheep shearer. Wool and hair samples were collected in sterile labeled plastic bags from different body parts of the animals i.e., abdomen, neck + face, fore limbs and hind limbs. After collection, samples were transported to the Central Veterinary Diagnostic Laboratory Tandojam and stored at –4°C till analyzed for B. anthracis spores.
Processing of Samples
Extraction of B. anthracis spores was carried out from wool and hair samples according to OIE manual (OIE, 2004). In brief, a 2.5 g sample (wool or hair) sample was suspended in 100 ml sterile distilled water. Supernatants were sieved through cellulose nitrate membrane (0.45 μm, Whatman International Ltd., England) and deposits were suspended in 5 ml sterile phosphate buffer saline (pH 6.6 ± 0.1). The aliquot was heated at 70°C for 20 min in water bath to kill all bacteria other than spores, and then centrifuged at 5000 rpm for 20 min. The sample was pelleted and resuspended in 2 ml phosphate buffer saline. Then, the aliquot was used to culture on Polymyxin B - Lysozyme - EDTA - Thallous acetate agar (PLET agar, Sigma-Aldrich, Switzerland), a selective media for growth of B. anthracis. For confirmation, the colonies were further grown on different culture media plates like blood agar, Brain Heart Infusion agar etc., to observe the characteristic morphology (Khan et al., 2016; WHO, 2008). Cultured plates were incubated aerobically at 37°C for 24–48 h. A sample was recognized as positive if at least a single colony was noticed on PLET agar. Moreover, the B. anthracis isolates were subjected to biochemical tests and susceptibility test against antibiotic penicillin-G (10 IU/disc; Difco, Labarotries Inc.) (Dragon et al., 2001; WHO, 2008).
Statistical Analysis
The data were analysed using JMP 5.0.1a software (SAS Institute Inc., Cary, NC, USA). One way ANOVA was applied to calculate the statistical differeces between different age-groups and body parts of animals. A p value < 0.05 was considered statistically significant.
Results and Discussion
Incidence of B. anthracis Spores in Wool and Hair Samples of Small Ruminants
The present study has investigated for the first time the incidence of B. anthracis spores on the body of small ruminants, i.e., sheep and goat in district Tharparkar, Sindh. As shown in Figure 1, out of 40 sheep wool samples, 12 (30%) were positive while remaining 28 (70%) were declared negative, however, among 40 goat hair samples 10 (25%) were positive while remaining 30 (75%) were found negative. The overall incidence of B. anthracis spores in wool and hair was recorded 28% (22/80). The statistical analysis showed a significant difference (P < 0.05) of anthrax spores occurrence between sheep and goat.
The spores of B. anthracis are extremely resistant to environmental factors and during unfavourable conditions may exist in dormant state for hundreds of years between epidemics (Ahsan et al., 2013). Tharparkar is a drought area of Sindh province that has high temperature and humidity (about 40 °C and 75%, respectively) throughout the year, which probably favours the high survival rates of anthrax spores (Banerjee, 2011). These environmental conditions could be linked with many sporadic outbreaks of anthrax in the Tharparkar that has been reported by the local veterinrians in last decade (unpublished data). Our current study has demonstrated 28% overall incidence of B. anthracis spores on the body of small ruminants in district Tharparkar, which are in agreement with previous study of Ahsan et al. (2013) who reported a 29.17% prevalence of anthrax spores in soil of Sirajganj, Bangladesh. Small ruminants’s hair usually get endospores from soil and long wavy hairs of sheep provide more protection and surface area for spores attachment, thus sheep may harbor more spores than goat.
Incidence of B. anthracis Spores at Different Body Parts of Sheep and Goat
Currently, investigation was carried-out to record the incidence of B. anthracis spores in wool and hair samples, taken from different parts of the body such as abdomen, neck + face, fore limbs and hind limbs of sheep and goat and results are indicated in Table 1. A relatively high incidence was observed in abdomen samples (35%), followed by neck + face (30%), fore limbs (25%) and hind limbs (20%) of small ruminants.
From the positive sheep wool samples, the 4/12 (33.33%) positive samples were identified from abdomen, 2/8 (25%) from neck + face side, 3/12 (25%) from fore limbs and 3/8 (37.5%) from hind limbs samples. Similarly, among the 10 positive hair samples of goat, 3/8 (37.5%) were from abdomen, 4/12 (33.33%) from neck + face side, 2/8 (25%) from fore limbs, while 1/12 (8.33%) from hind limbs were found positive for B. anthracis spores (Table 1). The statistical analysis showed that the overall difference among different body sites, was statistically non-significant (P=0.8232). Similarly, the difference among different body sites for both sheep and goat was also found non-significant (P > 0.05).
These results show that anthrax spores are equally present on the various body sites of small ruminants. Some numerical differences recorded in present study could be related with animal behavior like a slight higher incidence in abdomen samples probably due to more contact of this body part with the ground (sitting position).
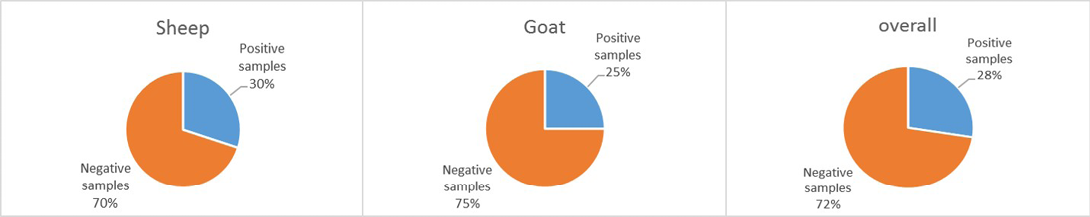
Figure 1: The incidence of Bacillus anthracis spores on the body of small ruminants in district Tharparkar
Table 1: The incidence of Bacillus anthracis spores on different body parts of small ruminants in district Tharparkar
|
Body sites |
Sheep |
Goat |
Overall Prevalence No. (%) |
||
|
Number of samples analyzed |
Positive samples No. (%) |
Number of samples analyzed |
Positive samples No. (%) |
||
|
Abdomen |
12 |
04 (33.33) |
08 |
03 (37.50) |
07 (35.00) |
|
Neck + Face |
08 |
02 (25.00) |
12 |
04 (33.33) |
06 (30.00) |
|
Fore limbs |
12 |
03 (25.00) |
08 |
02 (25.00) |
05 (25.00) |
|
Hind limbs |
08 |
03 (37.50) |
12 |
01 (08.33) |
04 (20.00) |
|
Overall |
40 |
12 (30.00) |
40 |
10 (25.00) |
22 (27.50) |
|
P- Value |
0.8810 |
0.5724 |
0.8232 |
||
Table 2: Age-wise prevalence of Bacillus anthracis spores on the body of small ruminants in district Tharparkar
|
Age groups |
Sheep |
Goat |
Overall Prevalence No. (%) |
||
|
Number of samples analyzed |
Positive samples No. (%) |
Number of samples analyzed |
Positive samples No. (%) |
||
|
2 years |
08 |
04 (50.00) |
12 |
06 (50.00) |
10 (50.00) |
|
2 ½ years |
08 |
03 (37.50) |
12 |
03 (25.00) |
06 (30.00) |
|
3 years |
12 |
03 (25.00) |
08 |
01 (12.50) |
04 (20.00) |
|
4 years |
12 |
02 (16.66) |
08 |
00 (0.00) |
02 (10.00) |
|
P- Value |
0.8810 |
0.0384 |
0.0952 |
||
It is well established that ferralic soils (sandy soil) consist more spores due to lowest moisture content than calcaric regosoils (loamy-clay soil) types (Cloete, 2013). It is probably due to lowest organic matter, carbon, moisture and pH contents of soil that have been associated with the presence of anthrax spores (WHO, 2008).
Prevalence of B. anthracis Spores in Different Age Groups of Sheep and Goat
The data regarding prevalence of anthrax spores in wool and hair samples collected from different age groups of sheep and goat has been depicted in Table 2. Among the positive samples of sheep, the highest prevalence was recorded in 2 years of age i.e., 4/8 (50%). Whereas, 3/8 (37.5%), 3/12 (25%) and 2/12 (16.6%) positive samples were identified in age groups of 2 ½, 3 and 4 years respectively. Likewise, among the positive samples of goat, the prevalence of B. anthracis spores was found higher in 2 years of age (6/12, 50%), followed by 2 ½ years (3/12, 25%) and 3 years (1/8, 12.5%) of age groups respectively. However, the incidence of B. anthracis spores was not found in age-group of 4 years of goat. The statistical analysis showed that the overall difference among the different age groups of small ruminants as well as individual differences for sheep was statistically non-significant (P > 0.05), however, in goat the difference among the different age groups was significant (P < 0.05).
In agreement to our results previous studies have also indicated the non significant associations of age for prevalence of bacterial diseases in farm animals (Kiros et al., 2016; Leghari et al., 2016). Shabbir et al. (2015) have also reported no association of risk factors with prevalence of B. anthracis DNA in soil samples analyzed from Lahore, Pakistan. Similar results were reported by a recent study in Belgium (Kissling et al., 2012).
In summary, the skin (wool and hair) of small ruminants was a habitat of B. anthracis spores. Sheep wool harbored relatively higher spore numbers as compared to goat hair. These results could be useful for control of anthrax in the area as well as for people dealing with small ruminants skins.
Acknowledgement
The study was fiancially supported through research assistance under the project “Pilot project on surveillance of Bovine Tuberculosis, Brucellosis and other zoonotic Diseases (eight district)” by the Central Veterinary Diagnostic Laboratory, Tandojam, Directorate of Veterinary Research and Diagnosis, Government of Sindh.
Conflict of Interest
The authors declare no conflict of interest.
Authors’ Contribution
Maryam Rajput conducted the research work and Asghar A. Kamboh was her supervisor. P. Dewani, and R. Rind were co-supervisors, while A. P. Umrani help in study design.
References






