Journal of Animal Health and Production
Review Article
Journal of Animal Health and Production 1 (1): 1–5Nucleotides Supplementation Improves Various Function of the Body
Umar Bacha1*, Muhammad Nasir1, Muhammad Asif Ali1, Javed Muhammad2, Ali Ahmad Sheikh2
- Department of Food Science & Human Nutrition, University of Veterinary & animal Sciences, Lahore, Pakistan
- 2University Diagnostic laboratory, University of Veterinary & Animal Sciences, Lahore, Pakistan
*Corresponding author: bacha.umar474@gmail.com
ARTICLE CITATION:
Bacha U, Nasir M, Ali MA, Muhammad J, Sheikh AA (2013). Nucleotides supplementation improves various function of the body. J Anim. Health Prod. 1 (1): 1–5.
Received: 2013–03–05, Revised: 2013–04–14, Accepted: 2013–04–20
The electronic version of this article is the complete one and can be found online at
(
http://nexusacademicpublishers.com/table_contents_detail/11/32/html
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
Nucleotides being novel substances act as prebiotic and; in most cases regulate function of gastro intestinal tract (GIT). They immensely regulate genes expression of immune cells, relieving stress, execute neurodevelopment and inhibit apoptosis. More so, they take part in oxido–reduction process, propagate cell signaling and regulate cell cycle of mammalian cells. Based on these important functions, European commission permitted the use of certain nucleotides in preparation of infant foods. However, nucleotides inclusion in the diet has to be elucidated for clinical significance particularly a threshold level of nucleotides inclusion has to be find out. Where levels of polyunsaturated fatty acid (PUFA) may not exceeds than monounsaturated fatty acid (MUFA) because of adverse effect associated with PUFA. Studies have shown promising results regarding elongation and concentration of various fatty acids. But still there is need to determine whether this effect may or not produce oxidative stress because optimum level of fatty acid is necessary to avoid stress particularly in animals.
Food beside its nutritional needs when provide substances aiming at promotion of health are known as functional food (Meyer, 2009). In recent years, such noval substances have been discovered and characterized. Among which one such class of functional substances are nucleotides (NTs). The NTs are proved to be possessing immense role in many of the biological reactions that are crucial for life sustenance of both human and animals. This review paper high lights important indices of nucleotides which offer significant role to the health at earlier stages of life.
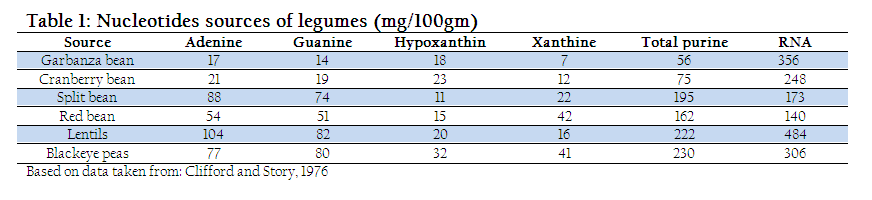
In milk, nucleotides are present as non–protein nitrogen (NPN substances) and nucleosides (Liao et al., 2011). The concentration of these NPN substances is higher at the beginning however, later on decreases with the passage of time. Beside milk, nucleoprotein and nucleic acids are the two major nucleotide sources found at different concentration in lamb, liver, and mushrooms (Dancey et al., 2006). Similarly, plants and legumes (Table. 1) also provide substantial amount of nucleotide (Clifford and Story, 1976). However, the quantity of nucleotide depends on various factors such as nature of milk for example colostrum which contains higher quantity of nucleotide and lactation period which are in inverse proportion with concentration of nucleotide (Schlimme et al., 2000).
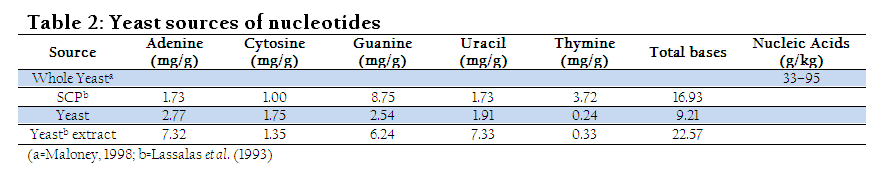
Being health friendly substances, European commission permitted the use of certain permitted supplementation in preparation of infant and follow–on formula (Schlimme et al., 2000). Yeast can also provide reasonable concentration of the nucleotides if appropriately grow and processed (Beluhan and Maric, 1995). Thus, its utilization in feed and food might be helpful to fulfill daily demand of the animals and human. Nucleotide derived from single cell protein (SCP) is seven times higher in concentration than meat (Ingledew, 1999). Diet with added nucleotides positively affects physiological indices of broiler chicken meat. Broiler chickens fed on nucleotide diet resulted in redder colour (P≤0.001) and higher mineral content in the meat (Chiofalo et al., 2011). The red colour of the meat might be due the utilization of nucleotides in DNA synthesis (especially expression of genes of myoglobin) which then make protein like myoglobin. However, scarcity in scientific literature still exists regarding this view and need research at molecular level to find out exact explanation. Surprisingly, nucleotides addition at 0.1% in broiler chicken diet improved unsaturated fatty acids (UAF) synthesis, growth and health (Mateo et al., 2004).
Health benefits of nucleotide depend on nucleotide doses and types. Nucleotide–fortified formula supplemented at concentration about 1.9 mg/ 418.4 kJ is sufficient. Similarly, nucleotides di containing 33 mg/ L may exert positive impacts on the body. However, to achieve more desirable outcome, 72 mg/L nucleotide is recommended (Chiofalo et al., 2011). Since, concentration of nucleotide in various formulation were different therefore, larger scale study is needed to optimize nucleotide level for each type of diet.
There are no specific channels for nucleotide absorption however; these are mostly absorbed through facilitated diffusion and specific Na+–dependent carrier–mediated mechanisms (Gutierrez–Castrellon et al., 2007) from upper small intestine. Under normal condition, nucleotides have limited access for transport because of its high negative charge which makes trouble in passing the lipid bilayer membrane. Also, its transport across the membrane needs ATPs to facilitate movement. Therefore, to reduce hindrance in transport, nucleotides are first converted into nucleoside which then can easily pass through lipid membrane.
Nucleotides after getting into blood stream store in enterocytes (Uauy et al., 1994). However, these cells stores little amount of nucleotide as compared to cancerous cells which stores greater quantity of nucleotide. This indicates that normal cells are dependent on constant supply of dietary nucleotides. In animal studies, 2 to 5% of dietary NTs are deposited in small intestinal, liver, and skeletal muscles tissue pools (Bronk and Hastewell, 1987). In fasting state and in young animals, NTs retention elevates in tissues ( Sanderson and He, 1994).
Bases for genetic information The most important function being attributed to NTs are its placement in genetic material as bases for DNA and RNA which control cellular functions of the body cells. Thus DNA and RNA uses four specific bases to represent living beings (Saviano and Clifford, 1978). Each nucleotide comprise of sugar, phosphate and a nitrogenous base which may be adenine guanine (purine) and cytocine, urasil, thyamine (pyrmidine) Individual nucleotide joined to each other through phosphodieter bond making nucleotide polymer in 3→5 direction. The invidual nucleotide in polynucleotide molecule is referred to as nucleotide. The overall charges on polynucleotide are negative (acidic) due to phosphate groups and therefore behave as polyannions at physiological pHs. Apart from NTs genetic placement, nucleotides also function as reservoir of energy such as ATPs. This facilitates cellular function like active and facilitated transport. The body can synthesis sufficient quantity of nucleotides and seems to be no need of the exogenous NT inclusion. However, it is also true that during rapid growth, it become conditionally essential (Gross and Saviano, 1991) and the term essential may be used when internal synthesis is either diminished or less efficient to fulfill body requirement.
Early days in animals and human life are very crucial because at this stage maximum growth and development occur. If at this stage proper nutrition is not fed to the infants then it may result in later on lower immunity, higher risk of infection and other diseases. Animals studies have shown that NT promotes growth particularly, immunity (Anandagopu et al., 2008). This observation gave clue that NT addition to human diet would also boost immunity in humans. As indicated by the “Adjuvant” property of NT in infants during vaccination. In infants (2 months old) fed on NT (33mg/L) supplemented formula showed higher natural killer (NK) cells production compared to breast fed infants (Maldonado et al., 2010). How NTs regulate gene expression is still in dormant stage however, NTs attach with cells receptors with help of transcription factors (Hawkes et al., 2006). Thus a complete reaction may need interaction between NT and transcription factors to express a particular gene. For example, intestinal genes of cytokine activated after NT intake (Gil, 2002). Hence, this indicated that immune cells not only activated against antigen but also proliferates including lymphocytes (cytotoxic T cells). Riera et al. (2013) reported improvements in immune response after NTs supplementation for four weeks.
Dietary NTs up regulates genes of NK cells to produce more interleukin (IL–6, IL–8) which have deleterious effects on pathogenic cells (Sanchez–Pozo and Gill, 2002). Nucleotide deficient diet resulted in less production of NK cells in mice spleen cells. These NK cells are believed to execute malignant cells therefore, are very important in inhibition of tumor propagation. In human trials women given NTs (1.0 mg/day) for four weeks reduced C reactive protein level (Sanchez–Pozo and Gill, 2002). Also, this diet resulted in increased T–lymphocytes (CD3+), T–lymphocytes helpers (CD4+) and suppressor/cytotoxic T–lymphocytes (CD8+) two folds. How NK cells destroy tumor cells are unclear but however, malignant cells produces “protein phosphatase 2A (PP2A–SET)” that acts as signaling molecules for activation of NK cells (Jyonouchi et al., 1996). Another possible mechanism might be the activity of macrophages which increases after NTs addition in the diet (Kocian and Kalanin, 1999). Both human and animals are naturally gifted with defense molecules against antigens. Antibodies (AB) are one such class of defense molecules. Diets with added NT enhanced production of humoral Ab against H. Infleuenza type b and diphtheria (Hawkes et al., 2006)
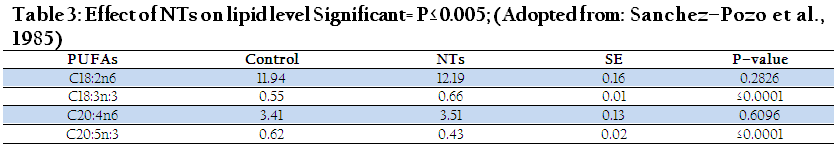
Fatty acids are the essential components of the human and animals tissues. Dietary NT have shown constructive role on lipids desaturation and elongation rates in fatty acids synthesis, especially long chain polyunsaturated fatty acids that have link with hyperactivity in children (Schlimme et al., 2000). Although, there are sufficient data available which states a case for long chain poly unsaturated (LCP) supplementation as a promising and potential treatment for learning disability but however, for more elucidation and definite role of LCP need research at larger scale in the future. Both omega 3 and omega 6 fatty acids (erythrocyte membrane of neonatal animals & new born) increased with NTs supplementation. Moreover, Nishizawa et al. (1996) reported cardiac friendly behavior of NTs. It would be imperative to carry out long term study to fully elucidate the role of NTs and its exploitation for obesity treatment which now a day stand one of the chronic human ailment.
Infected and mainly injured cells are unable to synthesis NTs from de novo pathway (Stasollaa et al., 2003). Therefore, such tissue optionally use salvage pathway, “a process in which cells uses NT resulted from the degradation of the DNA and RNA”. Tissue growth in response to NT diet has been confirmed in weanling rats. Intestinal epithelium is rather more active in tissue proliferation because these cells are comparatively more involved in active & passive transport of the molecules. Thus, require fast growth which results in high villi height and crypt depth (Domeneghini et al., 2004). However, some studies also showed that crypt depth remains unchanged after parenteral supplementation of nucleic acids (Tsujinaka et al., 1999). It is further supported by orotate, a pyrimidine precursor and uracil activator supplementation that activate jejunal growth in rats after bowl resection (SBR). Orotate 1% (wt/wt) is more potent for growth as compared to uracil 1% (wt/wt). Thus the use of orotate and uracil supplementation offer new opportunity in nutrition and thereby promotes adaptive growth (Evans et al., 2005).
Dietary NTs are reported to be multifunctional in their nature. It increases concentration of the insulin like growth factor (IGF–1) and insulin like growth factor binding protein (IGFBP–3) (Vasquez–Garibay et al., 2006). Therefore, IGF, a hormone known as somatomedin C with similarity in molecular structure to insulin might have role in enhancing growth. This hormone activates cell growth especially that of nerve cell proliferate cells and also, inhibits apoptosis (Programmed cell death). Enteral feeding of the NTs has cardinal effects on IGF–1 production. Thus low mucosal growth and low differentiation of these cells are due to malnutrition and is altered to positive growth with the aid of NTs supplementation (Ortega et al., 1995).
Kishibuchi et al. (1997) reported renowned effects of NTs on gastro intestinal tract function (GIT). They further noted that intracellular spaces in GIT mucosal cells become narrow in response to NTs feeding. The mentioned mechanisms thus decrease the flow of antigen from extracellular environment to the cell interior. Moreover, NTs decreases intestinal “Cathepsin” activity in the ileum (Tsujinaka et al., 1999). It is also worth to mention that cathepsin (gastric aspartyl protease) encoded by the gene (CTSE) in human function as Pepsin A. Cathepsin is a protinase excreted by the mucus epithelial cells of GIT. Interestingly, it is the first aspartic protinase expressed in the fetal stomach. It is the major cause of (more than half) gastric cancers. Immature GIT not only demand for higher content of DNA & RNA but also individual NTs for protective function of the GIT. Clinical trials have confirmed “Catch up growth” in infants (Vasquez–Garibay et al., 2006). Thus malnourished infants might have the opportunity to be progressed towards normal growth. Similarly, liver pool of the NTs also depends on availability of exogenous NTs (Lopez–Navarro et al., 1995). NTs function like prebiotics because it promote intestinal microflora. Therefore, exerts positive impacts on Biofido–bacteria population in the animals and humans in a similar way as infants fed on mother milk (Tanaka and Mutai, 1980). It increases level of Lactobacillus acidophilus and Bifidobacteria (Mateo et al., 2004). Finally, NTs increases maltase and lactase ratio thus plays pivotal role in GIT maturity.
Dietary habits have pronounced effects on behavior (Christensen and Pettijohn, 2001) For example, carbohydrates rich food especially derived from vegetables and fruits relieve depressiveness. On the other hand, there are foods and feeds that contribute in producing stress in humans and animals respectively. Nucleotide sufficient diets are used to decrease stress level especially those having poor dietary status (Vasquez–Garibay et al., 2006). Recent finding suggested that NTs decreases response of the hormones associated with physiological stress (Naughton et al., 2007).
It is not clear that NTs supplements possess anti–oxidative property per se. however; NTs supplements could nevertheless take part in the prevention of DNA breakage due to oxidative stress. Nucleotides potentially reduces oxidative stress aroused from higher intake of PUFA in pigs feed. Thus, dietary oxidative stresses (genotoxic effects) on immune cells, DNA/RNA might be reduced in animals (Yamamoto et al., 1997).
Although it will be hard to locate exact mechanism of how NTs are metabolized in growing infants. However, it is also apparent from literature that intestine of animals and humans possess digestive enzymes (Salobir et al., 2005). These digestive enzymes are capable of digesting RNAs to Cytidine, uridine and uric acids (in vivo). Laboratory analysis also showed that NPN of the tissue homogenate of fetal intestine increased when incubated with human milk. Brush boarder epithelium produces digestive enzymes, pancreatic juices and bile salts (Welker et al., 2011). Both, RNA & DNA are degraded into oligonucleotides by endonucleases whereas, phosphodiesterase enzyme degrade oligonucleotides into free nucleosides. However, enzyme phosphorylase converts nucleoside into bases and ribose–1–phosphate. Thus, enzymatic conversion of the polynucleotides produced bulk of “degaradtive pool products” that are rapidly absorbs from small intestine.
Cell signaling is important biological processes which integrate and keep smoothly whole process of the cells. Nucleotides involve in formation of, cyclic adenosine monophosphate (cAMP) and cyclic guanosine mono phosphate (cGMP) (Torres et al., 1998). These two molecules are the most important cells regulatory substances and therefore, known as “Secondary messenger”. Hematopoiesis is the process in which blood cellular components are formed. Dietary NTs stimulate this process by proliferating bone marrow cells (Morley et al., 1987). Interestingly, adenosine therapy (intravenous) is a potential vasodilator (Voet and Voe, 1995) thus, might be helpful in cardiac disease (Carver and Walker, 1995) and may also be helpful in asthma treatment.
REFERENCES
Anandagopu P, Suhanya S, Jayaraj V and Rajasekaran E (2008). Role of thymine in protein coding frames of mRNA sequences. Bioinformation, 2 (7): 304-307.
http://dx.doi.org/10.6026/97320630002304
PMid:18478084 PMCid:PMC2374375
Beluhan S and Maric V (1995). Optimization of the RNA content Reduction in Saccharomyces Cerevisiae. Prehrambeno-tehnol.biotehnol. Rev.33 (2-3): 85-90.
Bronk JR and Hastewell JG (1987). The transport of pyrimidines into tissue rings cut from rat small intestine. J Physiol. 382: 475-488.
PMid:3625557 PMCid:PMC1183036
Carver JD and Walker VA (1995). The role of nucleotides in human nutrition. Nutr. Biochem. 6: 58-72.
http://dx.doi.org/10.1016/0955-2863(94)00019-I
Chiofalo B, Presti VI, Voini GS, Dalessandro E, Chiofalo V and Liotta I (2011). Nucleotides in broiler chicken diet: effect on breast muscles quality. Czech. J. Food. Sci. 29: 308-317.
Christensen L and Pettijohn L (2001). Mood and carbohydrate cravings. Appetite, 36:137-145.
http://dx.doi.org/10.1006/appe.2001.0390
PMid:11237349
Clifford AJ and Story DL (1976). Levels of purines in foods and their metabolic effects in rats. Journal of Nutrition 106, 435-442.
Dancey CP, Attree EA and Brown KF (2006). Nucleotide supplementation: a randomised double-blind placebo controlled trial of IntestAidIB in people with Irritable Bowel Syndrome. Nutrition Journal, 5:16, doi: 10.1186/1475-2891-5-16.
http://dx.doi.org/10.1186/1475-2891-5-16
Domeneghini C, Di Giancamillo A, Savoini G, Paratte R, Bontempo V and DellOrto V (2004). Structural patterns of swine ileal mucosa following L-glutamine and nucleotide administration during the weaning period: A histochemical and histometrical study. Histol and Histopathol. 19: 49-58.
PMid:14702171
Evans ME, Tian J, Gu L, Jones H and Ziegler TR (2005). Dietary Supplementation With Orotate and Uracil Increases Adaptive Growth of Jejunal Mucosa After Massive Small Bowel Resection in Rats. J Parenteral and Enteral Nutr. 29 (5): 315-321.
http://dx.doi.org/10.1177/0148607105029005315
Gil A (2002). Modulation of the immune response mediated by dietary nucleotides. European J Clinical Nutr. 56 (S3): S1-S4.
http://dx.doi.org/10.1038/sj.ejcn.1601475
PMid:12142952
Gross CJ and Saviano DA (1991). The effect of nutritional state and allopurinol on nucleotide formation in enterocytes from the guinea pig small intestine. Biochem. Biophys. Acta. 1073: 260-267.
http://dx.doi.org/10.1016/0304-4165(91)90130-9
Gutierrez-Castrellon P, Mora-Magana I, Dıaz-Garcıa I, Jimenez-Gutierrez C, Ramirez-Mayans J and Solomon-Santibanez GA (2007). Immune response to nucleotide-supplemented infant formulae: systematic review and meta-analysis. British J Nutr. 98, Suppl. 1, S64–S67.
http://dx.doi.org/10.1017/S000711450783296X
PMid:17922963
Hawkes JS, Gibson RA, Roberton D, Makrides M (2006). Effect of dietary nucleotide supplementation on growth and immune function in term infants: a randomized controlled trial. Eur J Clin Nutr. 60 (2): 254-64.
http://dx.doi.org/10.1038/sj.ejcn.1602310
PMid:16234834
Ingledew WM (1999). Yeast-could you base a business on this bug? In Biotechnology in the Feed Industry. Proc. of Alltech's 15th Annual Symposium. T. P. Lyons and K. A. Jacques, eds. Nottingham University Press, Nottingham, UK. Pages 27-47.
Jyonouchi H, Sun S and Sato S (1996). Nucleotide-free diet suppresses antigen-driven cytokine production by primed T-cell: effects of supplemental nucleotides and fatty acids. Nutr. 12: 608-615.
http://dx.doi.org/10.1016/S0899-9007(96)00176-1
Kishibuchi M, Tsujinaka T, Yano M, Morimoto T, Iijima S, Ogawa A, Shiozaki H and Monden M (1997). Effects of nucleoside and a nucleotide mixture on gutmucosal barrier function on parenteral nutrition in rats. J. Parenter. Enteral Nutr. 21:104-111.
http://dx.doi.org/10.1177/0148607197021002104
Kocian J and Kalanin J (1999). Changes in cellular immunity after administration of nucleotides. Cas Lek Cesk, 138: 209-211.
PMid:10510536
Lassalas B, Jouany JP and Broudiscou L (1993). Dosage des bases puriques et pyrimidiques pcr chromatographie liqide a haunte performance. Annales de Zootechnie, 42, 170-171.
http://dx.doi.org/10.1051/animres:19930240
Liao KY, Wu TC, Huang CF, Lin CC, Huang IF, Wu L (2011). Profile of nucleotides and nucleosides in Taiwanese human milk. Pediatr Neonatol. 52 (2): 93-7.
http://dx.doi.org/10.1016/j.pedneo.2011.02.012
PMid:21524629
Lopez-Navarro AT, Gil A and Sanchez-Pozo A (1995). Deprivation of dietary nucleotides results in a transient decrease in acid-soluble nucleotides and RNA concentration in rat liver J. Nutr. 125: 2090-2095.
PMid:7543948
Maldonado J, Lara-Villoslada F, Sierra S, Sempere, Gomez M, Rodriguez JM, Boza J, Xaus J and Olivares M (2010). Safety and tolerance of the human milk probiotics strain Lactobacillus salivarius CECT5713 in 6-month-old children. Nutr. 26:1082–7.
http://dx.doi.org/10.1016/j.nut.2009.08.023
PMid:20018483
Maloney D (1998). Yeasts. in Kirk-Othmer Encyclopedia of Chemical Technology. 4th, ed. J. I. Kroschwitz and M. Howe-Grant, eds. John Wiley and Sons, Inc., New York, NY. Pages 761-788.
Mateo, C.D, Peters DN and Stein HH (2004). Nucleotides in sow colostrums and milk at different stages of lactation. J. Anim. Sci. 82:1339-1342.
PMid:15144074
Meyer R (2009). Infant feed first year 1: Feeding practices in the first six months of life. J Fam Health Care, 19:13-6.
PMid:19370861
Morley DJ, Hawley DM, Ulbright TM, Butler LG, Culp JS and Hodes ME (1987). Distribution of phosphodiesterase I in normal human tissues. J. Histochem. Cytochem. 35:75-82.
http://dx.doi.org/10.1177/35.1.3025290
PMid:3025290
Naughton ML, Bentley D and Koeppel P (2007). The effects of a nucleotide supplement on the immune and metabolic response to short term, high intensity exercise performance in trained male subjects. J Sports Med Phys Fitness. 47:112-8.
Nishizawa N, Harada Y and Fujimoto M (1996). Effect of dietary nucleotides on cholesterol metabolism in mice. Page 15 in Proc. 70th Annual Meeting of Japan Society for Bioscience, Biotechnology, and Agrochemistry. Tokyo, Japan.
Ortega MA, Nunez MC, Gil A and Sanchez-Pozo A (1995). Dietary nucleotides accelerate intestinal recovery after food deprivation in old rats. J Nutr. 125: 1413 – 1418.
PMid:7782893
Riera1 J, Pons V, Martinez-Puig D, Chetrit C, Tur JA, Pons A and Drobnic1 F (2013) Dietary nucleotide improves markers of immune response to strenuous exercise under a cold environment. J Int. Society of Sports Nutr. 10 (1): 20. Doi: 10.1186/1550-2783-10-20.
http://dx.doi.org/10.1186/1550-2783-10-20
Salobir J, Rezar V, Pajk T and Levart A (2005). Effect of nucleotide supplementation on lymphocyte DNA damaged induced by dietary oxidative stress in pigs. Animal Sci. 81: 135-149.
http://dx.doi.org/10.1079/ASC42290135
Sanchez-Pozo A and Gil A (2002). Nucleotides as semiessential nutritional components. British J Nutr. 87, Suppl. 1, S135–S137.
http://dx.doi.org/10.1079/BJN2001467
Sanchez-Pozo A, Pita ML, Martinez A, Molina JA, Sanchez-Medina R and Gil A (1985). Effect of dietary nucleotides upon lipoprotein pattern of newborn infants. Nutr. Res. 6: 53-57.
Sanderson IR and He J (1994). Nucleotide uptake and metabolism by intestinal epithelial cells. J. Nutr. 124:131-137.
Saviano DA and Clifford AJ (1978). Absorption, tissue incorporation and excretion of free purine bases in the rat. Nutr. Rep. Int. 17: 551-556.
Schlimme E, Martin D and Meisal H (2000). Nucleosides and nucleotides: Natural bioactive substances in milk and colostrum. British J Nutr. 84, Supple. 1, S59-S68.
http://dx.doi.org/10.1017/S0007114500002269
PMid:11242448
Stasollaa C, Katahira R, Thorped TA (2003). Hiroshi Ashiharab, Purine and pyrimidine nucleotide metabolism in higher plants. Journal of Plant Physiology, 160 (11): 1271–1295.
http://dx.doi.org/10.1078/0176-1617-01169
PMid:14658380
Tanaka R and Mutai M (1980). Improved medium for selective isolation and enumeration of Bifidobacterium. Appl. Environ. Microbiol. 40: 866-869.
PMid:7447440 PMCid:PMC291680
Torres MI, Fernandez MI, Gil A and Rios A (1998). Dietary nucleotides have cytoprotective properties in rat liver damaged by thioacetamide. Life Sciences, 62 (1): 13-32.
http://dx.doi.org/10.1016/S0024-3205(97)01033-3
Tsujinaka T, Kishibuchi M, Iijima S, Yano M and Monden M (1999). Nucleotides and intestine. JPEN J Parenter Enteral Nutr. 23 (5 Suppl):S74-77.
http://dx.doi.org/10.1177/014860719902300519
PMid:10483901
Uauy R, Quan R and Gil A (1994). Role of nucleotides in intestinal development and repair: Implications for infant nutrition. J. Nutr. 124 (8 S): 1436S-1441S.
Vasquez-Garibay E, Stein K, Kratzsch J, Romero-Velarde and Jahreis J (2006). Effect of nucleotide intake and nutritional recovery on insulin-like growth factor I and other hormonal biomarkers in severely malnourished children. British J Nutr. 96 (4): 683-90. PMid:17010227
Voet D and Voe JG (1995). Nucleotide Metabolism. in Biochemistry. 2nd ed., N. Rose, ed. John Wiley and Sons, Inc. New York, NY Pages 795-797.
Welker TL, Lim C, Akosy MY and Klesius PH (2011). Effects of dietary supplementation of a purified mixture on immune function and disease and stress resistance in channel catfish, ictalurus punctatus. Aquaculture Res. 42.
http://dx.doi.org/10.1111/j.1365-2109.2010.02794.x
Yamamoto S, Wang MF, Adjei AA and Ameho CK (1997). Role of nucleotides and nucleosides in the immune system, gut reparation after injury, and brain function. Nutr. 13: 372-374
http://dx.doi.org/10.1016/S0899-9007(96)00376-0