Journal of Animal Health and Production
Research Article
Immune Response, Health Status, Blood Components and Growth Performance of Suckling Calves Treated with Thymus vulgaris Extract
Wael Mohamed Wafa1*, Ashraf Awad Abd El-Tawab2, Fatma Ibrahim Elhofy2, Yasmeen Mamdouh Bedawy1
1Animal Production Research Institute, Agricultural Research Center, Giza, Egypt; 2Bacteriology, Immunology and Mycology Department, Faculty of Veterinary Medicine, Benha University, Egypt.
Abstract | Newly born male Friesian calves (n=15) with average live body weight (LBW) of 35.07±0.63 kg after calving were divided into three groups (5 in each). Calves were fed colostrum from their dams for the first three days of age then whole milk meals were fed and the starter plus good quality berseem hay were given after the second week of age. In the first group, calves had no treatment and considered as control (G1), while those in G2 and G3 were received an oral dose of Thymus vulgaris extract (THY) at levels of 20 and 40 mg per kg BW, respectively. Treatment lasted from 3 up to 105 d of age. Results show that THY at 40 mg/kg LBW increased (P<0.05) serum immunoglobulins (IgG, IgM and IgA), hematological parameters, serum total lipids and cholesterol, improved liver and kidney functions and health status of the calves, while decreased diarrheal incidence. In conclusion, oral administration with thyme extract at a level of 40 mg/kg body weight to Frisian calves, during the suckling period had beneficial effects on their immunity and health status, consequently improving their growth rate and live body weight, which could help farmers to raise the suckling calves for breeding or milk and meat production to relieve milk and beef lack in Egypt.
Keywords | Blood, Calve, Growth, Health, Immunity, Thymus vulgaris
Received | May 17, 2021; Accepted | May 21, 2021; Published | August 25, 2021
*Correspondence | Wael Mohamed Wafa, Animal Production Research Institute, Agricultural Research Center, Giza, Egypt; Email: drwailfatoh1973@hotmail.com
Citation | Wafa WM, El-Tawab AAA, Elhofy FI, Bedawy YM (2021). Immune response, health status, blood components and growth performance of suckling calves treated with thymus vulgaris extract. J. Anim. Health Prod. 9(3): 342-351.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.3.342.351
ISSN | 2308-2801
Copyright © 2021 Wafa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Livestock is an important part of the agricultural production system in any country, especially developing countries, and plays an important role in the national economy as well as in the development of millions of rural households (Yeshiwas and Fentahun, 2017). One of the main objectives of developing animal production by the year 2030 is increasing per capita animal protein consumption. Egyptian government is one of the largest importers of live cattle and beef as presented in the FAS (foreign agricultural service) Egypt (2017) report.
The first week of calf age is the most critical period of their life because it is associated with several health problems which lead to about 10% mortality rate. Diarrhea is one of the major reasons of mortality in newborn calves (Vandeputte et al., 2010; Cho and Yoon, 2014) that affects the livestock industry globally (Tajik et al., 2012). Different bacterial infections cause diarrhea (Radostits et al., 2010). For several years, antibiotics were excessive used not only to control pathogens but also for enhancement the calf growth rate (Santos et al., 2015). The overuse of antibiotics in order to reduce these pathogens has led to the phenomenon of multi-drug resistant bacteria (Boskovic et al., 2015).
There are several types of plants which are consider as drug sources because they have antibacterial, antiviral, antifungal, antioxidant and insecticidal properties due to their contents of essential oils (EOs). These plants have been examined as alternatives in animal production for improving growing performance parameters and the quality characteristics of the derived products (Burt, 2004; Simitzis and Deligeorgis, 2011). Essential oils (Eos) have therapeutic potential properties as they employed in treatment a variety of animal diseases, they act through stimulation of blood circulation, they reduce pathogenic bacterial counts and improve the immunity response by increasing the nutrient digestion and raising essential nutrients availability from intestine (Zeng et al., 2015). Also, it has the potential to decrease antibiotic usage without reducing performance or increasing mortality of dairy calves (Santos et al., 2015). Both cell-mediated and humoral immune response increased in growing calves fed on diet supplemented with medicinal plants (Lakhani et al., 2019).
Thyme (THY), Thymus vulgaris, is an annual plant, grows in many parts of the world and has grassy appearance. This plant is commonly used in folk medicine because it has several medical properties (expectorant, antitussive, broncholytic, anti-spasmodic, antihelminthic, carminative and diuretic). Several studies indicated that THY oil contains many biologically active chemical compounds (pinene, thymol and caryophyllene) which are used in various diseases (Lee et al., 2005; Boskabady et al., 2006) with minimal side effects (Al-Asmari et al., 2017). It contains volatile compounds and has been used in the treatment of many diseases in human and animals. This plant had anti-bacterial effect on E. coli (Khodaei et al., 2013), and was used in animal nutrition of calves (Seifzadeh et al., 2016a) to lower the number of diarrheal incidence in calves (Darabighane et al., 2016). The supplementation of THY in feedlot-calves finishing diet showed improvement in ruminal fermentation (Vakili et al., 2013). The oregano water (OW) that has high amount of carvacrol and thymol (it belongs to the same species as of the thyme) had potential effect on the level of immunoglobulins (IgA, IgG and IgM) in calves (Ozkaya et al., 2017).
The aim of the present study was to study the effect of thyme extract on growth performance, hematological and biochemical parameters, and immune status of newly born Friesian calves during the suckling period.
MATERIALS AND METHODS
Animals
Total of 15 newly born male Friesian calves with average live body weight of 35.07±0.63 kg from the birth up to the weaning age (at 105 days) was used in this study. Animals were divided into three groups, five calves in each. All calves were free from any disease and with healthy appearance. The experimental calves were kept in individual pens (1.0 × 1.5 m) bedded with rice straw.
Feeding system
After parturition, calves were fed 1.5-2 kg of colostrum from their dams by feeding bottles within 30 min then they suckled whole milk up to the weaning age (105 d). Calves in the experimental groups were fed on the whole milk meals at 6 a.m. and 6 p.m. Cow milk was allowed from El-Gemmaiza Animal Production Research Station, Animal Production Research Institute, Agricultural Research Center.
The starter was given with good quality berseem hay after the second week of age (for all groups). Starter was allowed to calves from El-Marg Fodder Factory, Ministry of Agriculture and Land Reclamation. Berseem hay (BH; Trifolium alexandrinum) was allowed from experimental farm of El-Gemmaiza Research Station. Fresh water was available free at all day times. The starter used in this study was composed of 38% yellow corn, 23% soybean meal, 35% wheat bran, 2% molasses, 1% premix and 1% common salt. Calves were fed according to NRC (2001) recommendation.
Calves in all groups were fed on the same amount of milk, starter and berseem hay. Representative samples of milk (100 ml), starter (100 g) and berseem hay (200 g) were analyzed for dry matter (DM), crude protein (CP), ether extract (EE), crude fats (CF), nitrogen free extract (NFE) and ash contents (Table 1) according to the A.O.A.C. (1995).
Experimental design
The experimental calves were treated with THY extract (Camino Viejo de Pliego Km Alcantarilla, Murcia, ESPANA) at levels of 0, 20, and 40 mg per kg of body weight for the G1, G2, and G3, respectively (Ganjkhanlou et al., 2014). Calves in treatment groups (G2 and G3) were treated by adding the thyme extract (brownish powder and soluble in water) to the morning milk meal just prior to feeding. The experimental period lasted from 3 to 105 d of calf age (weaning age).
Handling and management of calves were conducted according to the Directive 2010/63/EU for animal protection that used for scientific purposes (Official Journal of the European Union, 2010).
Experimental procedures
The live body weight (LBW) of calves was recorded at 3
Table 1: Chemical analysis (on DM basis) of milk, starter and berseem hay fed to calves in all groups.
| Item* | Milk | Starter | Berseem hay |
| DM (%) | 12.90 | 91.17 | 90.07 |
| Chemical composition (%): | |||
| CP | 25.01 | 17.70 | 13.02 |
| CF | 0.000 | 6.050 | 27.41 |
| EE | 32.12 | 3.270 | 2.740 |
| NFE | 37.57 | 64.09 | 46.67 |
| Ash | 5.30 | 8.890 | 10.16 |
* DM: dry matter, CP: crude protein, CF: crude fiber, EE: ether extract, NFE: nitrogen free extract
(initial time), 30, 60, 90 and 105 (weaning) days of age, then the average total gain (TDG) was calculated at different age intervals. The calves in treatment groups (G2 and G3) were weighed weekly to determine the dose of THY treatment.
Health status scores of calves including respiratory and fecal scores were individually recorded biweekly to monitor their health status. Respiratory score was assigned on 1 to 3 scale as described by Bascom et al. (2002) where 1 was normal, 2 was a runny nose/eyes, and 3 was mucus discharge from nose/eyes and fever. Fecal scores were assigned as identified by Larson et al. (1977). Feces fluidity score was from 1 in normal to 4 in liquid case, feces thickness was from 1 in normal to 5 in viscous case, and faces smile was from 1 in normal to 3 in very bad smile case. Total health score was calculated by summation of respiratory scores, feces fluidity, thickness, and smile.
Blood sampling
Blood samples were taken after three hours of morning feeding (10 ml) from the jugular vein of calves in each group pre-treatment (0 time) and weaning. Test tubes with EDTA or without anticoagulant were used in blood collection. Blood samples without EDTA were allowed to clot, and Low-Speed Desktop Centrifuge (Bio-Lion: XC50S3) was used for centrifugation of blood at 3000 rpm for 20 min to separate the blood serum, which was stored at -20oC till blood biochemicals analysis.
Analytical methods of blood
In blood samples with EDTA, heamocytometer supplied with two cover slides (Sigma- Aldrich Bright-Line™: Z359629) was used for count of red blood cells (RBCs) and white blood cells (WBCs) using microscope (Swift SW350T) according to Verso (1964). Mission® Plus kit (REF C132-3031, USA) was used for determination of hemoglobin (Hb) concentration and packed cell volume (PCV %) according to Henry (2001).
Spectrophotometer (JENWAY 6405 UV/Vis, England) was used for measurement of blood serum biochemicals using commercial kits (Bioassay systems, San Francisco, USA) to determine total proteins (Henry, 1974), albumin (Doumas et al., 1971), total lipids (Zollner and Kirsch, 1962), cholesterol (Richmond, 1973), triglycerides (McGowan et al., 1983), urea (Bull et al., 1991), and creatinine (Bartles et al., 1972) concentrations as well as aspartate transaminase (AST) and alanine transaminase (ALT) activities. The globulin concentration was calculated by subtraction of serum albumin from total proteins concentration.
Immunoglobulins (IgG, IgM and IgA) concentrations in blood serum samples were determined using the quantitative ELISA (Bovine IgG, IgM, and IgA ELISA quantitative kit, Bethyl laboratories, UK) as described by Killingsworth and Savory (1972). The activity of AST and ALT transaminases were determined in blood serum according to Reitman and Frankel (1957).
ELISA plate reader, 96 Wells Microwell Plate and ELISA kits (Bovine IgG, IgM, and IgA ELISA Quantitative kit, Bethyl laboratories, UK) were used for determination of serum IgM, IgA and IgG concentrations.
Statistical analysis
Using SPSS analysis program (IBM SPSS version 25, 2017), the data of the present study were analyzed using one way-ANOVA. The significant differences were detected using Duncan Multiple Range Test (Duncan, 1955) and tested at P<0.05.
RESULTS
Immunity parameters
Blood plasma immunoglobulins: Results revealed insignificant differences in concentration of IgG, IgM, and IgA in blood serum of calves in the experimental groups within three days after calving. Although all types of immunoglobulins showed inconsistent trend of change during the suckling period, their values were the highest (P<0.05) in G3, moderate in G2, and the lowest in G1 (Figure 1).
Table 2: Effect of thyme extract on serum immunoglobulins values of calves at weaning relative to initial and control values.
| Item | G1 | G2 | G3 |
| IgG values at weaning | |||
| Relative to initial values (%) |
-24.21a |
-19.17a |
-5.10b |
| Relative to control group (%) | 100 | 107.49 | 126.54 |
| IgM values at weaning | |||
| Relative to initial values (%) |
-43.56a |
-9.76b |
0.00c |
| Relative to control group (%) | 100 | 162.28 | 177.19 |
| IgA values at weaning | |||
| Relative to initial values (%) |
116.13b |
121.21b |
168.75a |
| Relative to control group (%) | 100 | 108.96 |
128.36 |
a, b and c: Means denoted with different superscripts in the same row are significantly different at P<0.05.
Table 3: Effect of thyme extract treatment on hematological parameters of calves in the experimental groups.
| Item* | Period | G1 | G2 |
G3 |
| Hb (g/dl) | Initial | 10.90±1.00 | 10.73±0.96 | 10.65±0.75 |
| Weaning |
7.88±0.52b |
8.45±0.32b |
10.20±0.41a |
|
| PCV (%) | Initial | 32.06±2.95 | 31.55±2.81 | 31.40±2.21 |
| Weaning |
22.84±1.37b |
24.79±3.32ab |
30.06±0.46a |
|
|
RBCs (106/mm3) |
Initial | 8.49±0.86 | 8.33±0.86 | 8.40±0.86 |
| Weaning |
9.83±0.36b |
10.23±0.71ab |
11.95±0.66a |
|
|
WBCs (103/mm3) |
Initial | 11.05±0.85 | 10.95±0.97 | 10.93±1.04 |
| Weaning |
12.00±0.44a |
11.18±0.46ab |
10.25±0.55b |
a and b: Means denoted with different superscripts in the same row are significantly different at P<0.05.
* Hb: hemoglobin, PCV: packed cell volume, RBCs: red blood cells, WBCs: white blood cells
Supplementation of THY (40 mg/kg body weight) resulted in the lowest (P<0.05) reduction in IgG and IgM and the highest increase in IgA at weaning relative to initial values after calving. Also, values of all types of immunoglobulins at weaning were higher relative to control calves (Table 2).
Hematological parameters
Averages of all hematological parameters (Hb, PCV, RBCs and WBCs) showed insignificant differences among the experimental groups at the start of experiment (initial values). At weaning, Hb concentration was higher (P<0.05) in G3 than in G1 and G2. However, count of RBCs and PCV increased (P<0.05) and WBCs decreased (P<0.05) in G3 compared with G1, but did not differ significantly (P<0.05) from that in G2 (Table 3).
Liver function
Data in Table 4 revealed that the differences in concentration of serum total proteins (TP), albumin (AL), globulin (GL), and AL/GL ratio as well as activity of AST and ALT after calving, as markers of liver function, were not significant. At weaning, TP concentration increased (P<0.05), while AST and ALT activities decreased (P<0.05) in G3 in comparing with G1.
Kidney function
The differences in concentration of serum creatinine and urea after calving were not significant. At weaning, concentration of creatinine decreased (P<0.05), while urea increased (P<0.05) in G3 in comparing with G1 and G2 (Table 5).
Lipid profile
There were no significant differences in concentration of serum total lipids (TL), total cholesterol (TC), and triglycerides (TG) after calving. At weaning, TL and TC concentrations increased (P<0.05) in G3 in comparing with G1, but did not differ from that in G2 (Table 6).
Health indices
The present result of scores of feces fluidly and feces smile were lower (P<0.05) in G3 than in G1, but did not differ significantly from that in G2. Respiratory score was lower in G3 and G2 than in G1, while feces thickness score was not affected by THY treatment. These results reflected the lowest total health score in calves of G3, followed by G2, while the highest score was in G1 (Figure 2).
Table 4: Effect of thyme extract treatment on liver function of calves in the experimental groups.
| Item* | Period | G1 | G2 | G3 |
| Total protein (g/dL) | Initial | 7.14±0.14 | 7.31±0.15 | 7.44±0.17 |
| Weaning |
5.51±0.14b |
5.72±0.12ab |
5.97±0.08a |
|
| Albumin (g/dL) | Initial | 4.19±0.17 | 4.30±0.20 | 4.38±0.11 |
| Weaning | 3.21±0.15 | 3.24 ±0.03 | 3.35 ±0.11 | |
| Globulin (g/dL) | Initial | 2.94±0.09 | 3.02±0.10 | 3.06±0.21 |
| Weaning | 2.30±0.12 | 2.48±0.14 | 2.62±0.11 | |
|
AL/GL ratio |
Initial | 1.43±0.09 | 1.44±0.10 | 1.46±0.11 |
| Weaning | 1.41±0.10 | 1.32±0.08 | 1.29±0.09 | |
| AST (IU/L) | Initial | 68.25±1.55 | 68.50±2.33 | 67.75±3.75 |
| Weaning |
81.75±2.56a |
75.75±4.78ab |
66.00±2.27b |
|
| ALT (IU/L) | Initial | 18.55±0.62 | 18.28±0.95 | 18.35±0.72 |
| Weaning |
23.05±0.61a |
21.85±0.66a |
19.38±0.84b |
a and b: Means denoted with different superscripts in the same row are significantly different at P<0.05.
* AL/GL: albumin to globulin ratio, AST: aspartate transaminase, ALT: alanine transaminase
Table 5: Effect of thyme extract treatment on kidney function of calves in the experimental groups.
| Item | Period | G1 | G2 | G3 |
| Creatinine (mg/dL) | Initial | 1.62±0.33 | 1.73±0.35 | 1.52±0.25 |
| Weaning |
1.82±0.17a |
1.69±0.27a |
0.95±0.16b |
|
| Urea (mg/dL) | Initial | 8.35±0.34 | 8.26±0.50 | 8.40±0.36 |
| Weaning |
12.61±0.43b |
13.15±0.33b |
14.33±0.27a |
a and b: Means denoted with different superscripts in the same row are significantly different at P<0.05.
Table 6: Effect of thyme extract treatment on lipid profile in serum of calves in the experimental groups.
| Item | Period | G1 | G2 | G3 |
| Total lipids (g/dL) | Initial | 76.40±1.96 | 78.01±1.67 | 77.64±1.56 |
| Weaning |
70.56±1.41b |
73.52±1.60ab |
76.73±1.27a |
|
| Total cholesterol (mg/dL) | Initial | 102.63±1.87 | 101.88±2.52 | 102.15±1.96 |
| Weaning |
83.88±2.55b |
89.10±2.11ab |
95.48±2.10a |
|
| Triglycerides (mg/dL) | Initial | 103.95±5.44 | 114.93±5.85 |
109.35±7.60 |
| Weaning | 87.40±5.95 | 93.10±2.68 |
97.35±4.74 |
a and b: Means denoted with different superscripts in the same row are significantly different at P<0.05.
Table 7: Effect of thyme extract on total and daily weight gain of calves in the experimental groups during the suckling period.
| Item | Period | G1 | G2 | G3 | |
| Total weight gain (kg/animal) |
46.60±1.72c |
59.80±3.12b |
74.40±3.26a |
||
| Daily weight gain (kg/h/d) |
0.443±0.02c |
0.570±0.03b |
0.710±0.03a |
||
| Weight gain relative to control (%) | 100 | 128.3 | 159.7 | ||
a, b and c: Means denoted with different superscripts in the same row are significantly different at P<0.05.
Growth performance of calves
Live body weight: The present results illustrated in Figure 3 indicated that the differences in live body weight (LBW) of calves were not significant (P<0.05) at the beginning of experiment (3 d after calving, 0 time). Average LBW of calves was the highest in G3 as compared to other groups at all intervals of the suckling period. At weaning, LBW was the highest in G3, moderate in G2, and the lowest in G1.
Body weight gain: Results presented in Table 7 showed that total and daily weight gain of calves was the highest (P<0.05) in G3 compared with G2 and G1 during the entire length of the suckling period. However, calves in G2 showed moderate values, while those in G1 showed the lowest values.
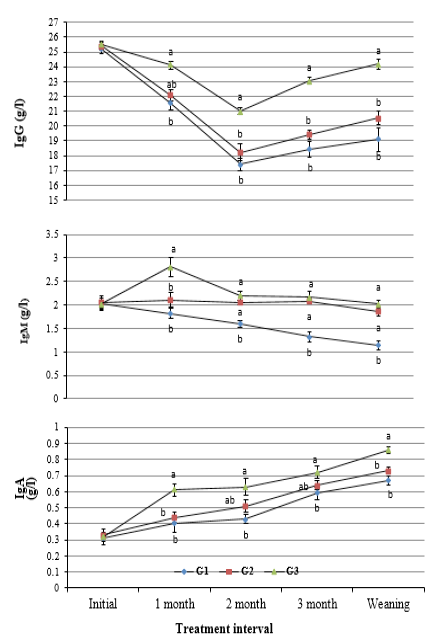
Figure 1: Change in concentration of IgG, IgM and IgA in blood serum of calves in the experimental groups during the suckling period. (a and b: Significant group differences at P<0.05)
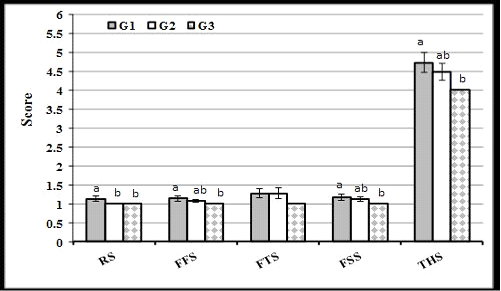
Figure 2: Effect of thyme extract treatment on average score of respiratory (RS), feces fluidity (FFS), feces thickness (FTS), feces smile (FSS), and total health (THS) of calves in experimental groups during the suckling period. (a and b: Significant group differences at P<0.05)
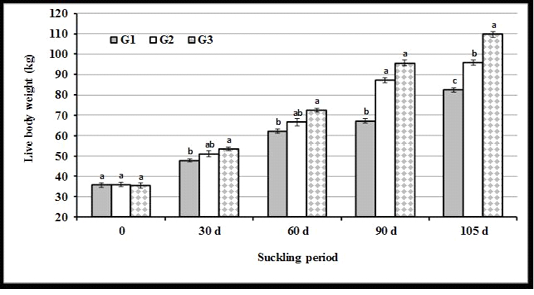
Figure 3: Live body weight of calves in experimental groups at different months of the suckling period. (a, b and c: Significant group differences at P<0.05)
DISCUSSION
Some authors used the Thymus vulgaris (THY) plant in animal nutrition to evaluate its effects on growth performance and immunity of calves (Seifzadeh et al., 2016a). They found marked effects of THY plant on live weight, gain, and immunity of calves during the suckling period. The present study aimed to evaluate different levels of THY extract on growth performance, immunity, blood biochemicals, and health status of Friesian calves during a normal suckling period of 105 days. As a strategy in dairy farms in Egypt, calves were targeted to wean at 105 d of age or when they reach to LBW of 85 kg/calf. In our study, calves in the control group (G1) without treatment reached an average of 82.4 kg at the end of weaning period versus 95.8 and 109.8 in G2 and G3, respectively. Accordingly, LBW of calves treated with TYM at a level of 20 mg/kg B.W. (G2) was higher by about 16.3% as compared to controls. When THY treatment level was increased to 40 mg/kg B.W., this increase reached to 33.2% in comparison with controls. These findings were reflected in higher weight gain by 128.3 and 159.7% for both THY treatment levels, respectively. Furthermore, these results may suggest the weaning time of calves to be at earlier age at 90 days in G2 and G3. These results are in accordance with Khamisabadi et al. (2016), who found that the dietary addition of thyme (3% on DM basis) improved average daily gain in lambs. Oregano water (OW) contain a high amount of carvacrol (994.3 g/kg) and thymol (5.7 g/kg) as found in THY (Helander et al., 1998). In this respect, calves fed oregano water-supplemented milk replacer were weaned earlier than those in control group (Ozkaya et al., 2017). Also, the dietary supplementation of herbal plants containing thyme (0.9%) significantly improved average daily gain of calves (Seifzadeh et al., 2016b). On the other hand, using essential oils of THY did not affect average gain and feed efficiency of calves fed high-concentrate diets (Vakili et al. (2013).
In association with improving LBW and weight gain in G3, the obtained results also showed remarkable (P< 0.05) increase in the serum concentration of IgM, IgG and IgA in calves treated with THY extract (40 mg/kg) as compared to the control. Improving the immunity response as affected by THY treatment agreed with Ozkaya et al. (2017) in calves receiving milk replacer supplemented with OW. Increasing immunoglobulins concentration in G3 was in parallel with hematological parameters, in terms of increasing PCV percentage, RBCs count and Hb level, and decreasing WBCs count, which is in agreement with Kovács et al. (2015), who found that 3% dietary THY supplementation significantly increased RBCs count in rabbits, but Darabighane et al. (2016) reported that THY had no effect on WBCs count in calves. Improving the immunoglobulins and hematological parameters may explain the superiority of growth performance of calves in G3 in comparing with G2 and G1.
Concerning the effect of THY on liver function, calves receiving THY extract in G3 showed the highest concentrations of total proteins, and the lowest AST and ALT activities, but albumin and globulin concentration, and even albumin to globulin ratio were not affected at weaning as compared to G1 and G2. These results are in agreement with increasing total proteins in rabbits treated with 3% THY (Kovács et al., 2015) and in birds (Abd El-Ghaney et al., 2017). Also, a medical plant mixture containing thyme had no significant effect on blood albumin concentration in calves (Seifzadeh et al., 2016b). The significant decrease in blood ALT and AST activities in calves in G3 disagreed with Vakili et al. (2013), who found that plasma activity of AST and ALT were not changed by THY. On the other hand, THY treatment improved kidney function of calves in G3, in terms of marked reduction in serum creatinine and increased urea concentration as compared to G1 and G2. Unfortunately, the available references on the effect of THY on blood creatinine level are scar although Abd El- Ghaney et al. (2017) found that serum creatinine content was not affected by THY in chicks at the 3rd and 5th weeks. The increase in urea concentration in G3 agreed with the results of Kovács et al. (2015), who found that 3% THY powder significantly increased urea concentration in growing rabbits. Contrary, Vakili et al. (2013) found that essential oil of THY did not affect urea concentration in calves fed high-concentrate diets. These finding may indicate that THY extract had no adverse effects on the liver and kidney functions of calves during the suckling period.
Regarding the effect of THY on lipid profile, total lipids and total cholesterol concentration increased, while triglycerides concentration was not affected in G3 compared with G1 and G2 at weaning. In comparable with the present results, Vakili et al. (2013) reported that plasma concentrations of cholesterol and triglycerides was not affected by feeding THY. Despite these effects of THY, the present values of total lipids, cholesterol, and triglycerides are within the normal ranges of growing calves (Pysera and Opalka 2001; Bozukluhan et al., 2017), which may suggest a save usage of THY as a treatment of the calves during the suckling period.
The observed improvement in immunity, liver and kidney functions of calves in G3 reflected the lowest scores of respiratory, feces fluidly, feces smile and total health, which may indicate an improvement in the health status and decrease of diarrheal incidence in calves. Generally, essential oils have therapeutic potential properties as they employed in treatment a variety of animal diseases; they act through stimulation of blood circulation, and improve the immune response and blood biochemical by increasing the nutrient digestion, raising essential nutrients availability from the intestine, and reducing pathogenic bacterial counts (Zeng et al., 2015). In this respect, antibacterial properties of THY essential oil to lower the number of diarrheal incidences in calves by decreasing E. coli count were reported by Darabighane et al. (2016). Also, Ozkaya et al. (2017) mentioned that OW in milk replacer with aromatic oregano improved the health status of Holstein-Friesian calves during the suckling period.
It is of interest to note that calves in G2 receiving a lower dose of THY showed some improvement in serum IgM concentration and respiratory score as compared to those in G1 (control). This trend reflected insignificantly heavier calves in G2 than in G1 at weaning. These findings may suggest that the effect of THY is dose depending manner
Anyway, the antibacterial activity of THY oil was due to thymol and carvacrol, which have inhibitory effects on the growth of enteric bacteria E. coli (Helander et al., 1998). The essential oil of THY also contain borneol, geraniol, and a variety of flavonoids such as thymonin, luteolin, naringenin and apigenin that acted as antimicrobial agent (Stahl-Biskup, 1991). Also, the essential oils enable to breakdown the lipids of the cell membrane of the bacteria because of their hydrophobicity characteristics and leading to cell structures disturbing and making them more permeable (Sikkema et al., 1994). Several researchers indicated that plant is one of drug sources because it have antibacterial, antiviral, antifungal, antioxidant and insecticidal properties which are due to their contents of essential oils that examined as alternatives animal production by improving growing performance and the quality of derived products (Burt, 2004; Simitzis and Deligeorgis, 2011).
CONCLUSION
Based on the aforementioned results, the current study concluded that the administration of Frisian calves with extract from thyme, at a level of 40 mg/kg body weight, during the suckling period (for 105 days) had beneficial effects on their immunity and health status, consequently improving their growth rate and live body weight to reach heavier weights at weaning or to wean calves at early ages. These findings may helpful for farmers to keep calves instead of their disposal by sell, which could help to relive the shortage of milk and beef in Egypt.
acknowledgements
The authors thank the El-Gemmezah Animal Production Experimental Station, APRI, Egypt for allowing animals that used in this study and also, thank the Clinical Pathology Department, Animal Health Research Institute, Tanta Laboratory for provide analytical chemicals and equipment.
Conflict of interest
The authors declare no conflicts of interest.
authors contribution
Wael Mohamed Wafa, Ashraf Awad Abd El-Tawab, Fatma Ibrahim Elhofy contributed to design the experimental work. Wael Mohamed Wafa, Yasmeen Mamdouh Bedawy conducted the experimental procedures and collected data. Fatma Ibrahim Elhofy, Yasmeen Mamdouh Bedawy performed the sample preparations for laboratory analysis. Wael Mohamed Wafa conducted the statistical analyses and critically revised the manuscript.
REFERENCES






