Journal of Animal Health and Production
Research Article
Efficacy of Disinfection on Airborne and Waterborne Fungal Load in Broiler Chicken Houses
Aya Nasser Ibrahim1, Hanan S. Khalefa1*, Hassan Aboul-Ella2, S. T. Moubarak1
1Department of Hygiene and Animal Management, Faculty of veterinary medicine, Cairo University, Egypt 12211; 2Department of microbiology, Faculty of veterinary medicine, Cairo University, Egypt 12211.
Abstract | Several studies have looked at the bacterial population in poultry houses, but there have been few papers on mycological contamination; especially isolation of fungal isolates from cooling pads’ water. As a consequence, this research aimed to isolate and identify airborne and waterborne fungi from a variety of surfaces in two closed broiler chicken housing in the Giza government. This study was carried out during the flocks’ growth period (on day 10, day 30, and two hours after disinfection). Twenty water samples were collected from water lines (at the entrance and from the ends) and cooling pads. While the 30 dust samples were collected from fans and the floor to isolate airborne fungi. Following the determination of the total fungal counts, macroscopical and microscopic identifications were done. Seven fungal species belong to five separate fungal genera were isolated; Aspergillus flavus (100%), Aspergillus niger (100%), Aspergillus fumigatus (87.5%), Mucor sp. (87.5%), Penicillium sp. (75%), Fusarium sp. (37.5%), while Dematiaceous sp. (25 %) was isolated only from the waterline and the fans in house 2. Total fungal count levels were found to be lower after disinfection. Furthermore, some of them survive after disinfection. However, since fungal pollution affects the safety of the environment and drinking water, effective disinfection procedures must be used.
Keywords | Broiler chicken houses, Fungal Contamination, Cooling pads, Dust, Antifungal disinfection
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | April 17, 2021; Accepted | June 22, 2021; Published | July 28, 2021
*Correspondence | Hanan Saad Khalefa, Department of Hygiene and Animal Management, Faculty of veterinary medicine, Cairo University, Giza, Egypt 12211; Email: hanansaad04@gmail.com
Citation | Ibrahim, A.N., Khalefa HS, Aboul-Ella H, Mubarak ST (2021). Efficacy of disinfection on airborne and waterborne fungal load in broiler chicken houses. J. Anim. Health Prod. 9(3): 296-302.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.3.296.302
ISSN | 2308-2801
Copyright © 2021 Ibrahim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The poultry industry is one of Egypt’s most important agricultural sectors, contributing a significant portion of the country’s animal protein supply, which includes white meats and eggs (El Nagar and Ibrahim, 2007). Despite its rapid progress, it faced several challenges, the most severe of which are infectious and non-infectious diseases (Bhuvaneswari, 2008; Espinosa et al., 2020). Stocking density in poultry farms is incredibly high in intensive production systems, making maintaining optimum microclimate and hygienic conditions difficult. Fungi and mycotic infections are prevalent in all forms of poultry productions, although they are less common than bacterial and viral infections.
Fungi are often present in the atmosphere, with varying concentrations depending on the climate as temperatures, humidity, and particulate emissions (Karwowska, 2005; Vučemilo et al., 2008). Aspergillus fumigatus, Fusarium sp., Penicillium sp., and Mucor sp. can be present in the dirt, feed, dust, and bedding, but not as much as the birds themselves (Kasprzyk, 2008). Many fungal spores are known as bioaerosols due to their limited small size. Molds from the genera Aspergillus, Candidiasis, Cladosporium, Penicillium, Alternaria, Scopulariopsis, Rhizopus, Mucormycoses, Histoplasmosis, and Cryptococcosis are often found in the fungal aerosol in rearing houses (Jo and Kang, 2005; Popescu et al., 2019). When it contributes to pathogenic microorganisms in water, fungi were traditionally neglected.
Fungi cause disease in two ways: they invade, damage, and kill the host’s body tissues, and they develop pathogenic signs and lesions of disease by releasing toxins known as mycotoxins. Aspergillosis and Candidiasis have a major impact on broiler health, while Histoplasmosis and Cryptococcosis have a public health risks. Lengthy or sustained exposure to high levels of respiratory fungal spores in a variety of agricultural settings has been linked to decreased lung capacity, as well as adverse effects such as asthma and inflammatory alveolitis, which are commonly referred to as farmer’s lung syndrome (Schierl et al., 2007). Moreover, Trichophyton and Cryptococcus species, for example, are among the many zoonotic yeasts and molds. They spread by contact, absorption, or inhalation, and valid waterborne pathways can be shown at a certain level. The diseases also cause diarrhea and compromise growth performance in broilers (Dhama et al., 2013).
Our research aimed to isolate and identify fungal population from two broiler house environments (drinking water lines, water of cooling pads, and dust) during the grow-out period, as well as evaluating the anti-fungal efficacy of the terminal disinfection.
MATERIALS AND METHODS
Broiler houses
In two closed broiler chickens houses in Giza, Egypt, microbiological analyses were conducted to determine air and water fungal contaminations throughout the grow-out period, as well as following the terminal disinfection of each house at autumn season. Each house was 100 m ×12 m in dimensions, with a capacity of 12500 birds per house; reared on a deep litter system. Artificial ventilation was used for air exchange. The birds were fed using an automated feeder system.
Sampling
A total of 20 water samples were collected from the drinking waterlines (at entrances and ends) and from the water of cooling pads, according to the methods mentioned by Maes et al. (2019). Furthermore, 30 dust samples were collected from fans and the floor using a sterile brush; samples from each site were pooled to get the original sample. During the grow-out period, samples were taken from each poultry house on days 10 and 30. Also, after 2 hours from terminal disinfection, samples from the same surfaces were collected using sterile swabs containing sterile saline. All water, dust, and swab samples were transported to the laboratory in a 4 ºC icebox for further examination and isolation of fungi.
Fungal isolation and identification
To obtain mold counts, one ml of the collected samples were subjected to ten-fold serial dilutions up to the fourth dilution level and plating on Sabouraud’s Dextrose Agar (SDA) with chloramphenicol. The plating was done in duplicate at each dilution stage to calculate the average fungal count. At 20-25 degrees Celsius, the plates were incubated for 3-5 days (McGrath, 1999; Mentese et al., 2017). After incubation, the fungal colonies were examined macroscopically and microscopically on a wet mount technique slide coated with lactophenol cotton blue dye and examined under the microscope with a 40x lens (Parija and Prabhakar, 1995).
Cleaning and disinfection of different poultry house surfaces
Cleaning and disinfection was done after the end of the grow-out period. The cleaning procedure included the following steps: (First: Dry cleaning, secondly: High-pressure wet washing of water and alkaline detergent and finally washing with clean water). These steps are followed by terminal disinfection, which varies depending on location: Waterline disinfection was performed using a highly concentrated disinfectant solution, strong acids (using a commercial product containing phosphoric acid at the recommended concentration), while fans and floors are disinfected with an aldehyde (BioShield, it is straw-colored liquid blend of glutaraldehyde and quaternary ammonium compound. Disinfectant at a recommended 1 liter of diluted disinfectant).
Statistical analysis
Results of total fungal counts (TFC) were summarized as means±SD, while isolation rates of fungal isolates were expressed as percentages. Data analyses were performed using Microsoft Office Excel 2010.
RESULTS AND DISCUSSION
Total fungal count (TFC) in different poultry house sources
In the current study, the prevalence of fungal contaminants was identified in different poultry house environment samples (drinking waterlines, water of cooling pads, and dust from fans and floor) (Table 1). The contamination by total fungal count in water lines reached the highest level in the entrance of water (18.00 ± 2.83 and 26.00 ± 18.38 CFU ×104 / 20 cm2) in house 1 and house 2, respectively. While, the average TFC at the two ends of water line systems recorded (10.00 ± 3.53 and 19.25 ± 18.74 CFU ×104 / 20 cm2), respectively in same houses. Results from cooling pads’ water showed high TFC at the end of rearing period reached to 26.30±33.51 CFU ×102/ ml compared to the first week 375±45 CFU/ml. Total fungal count (TFC) from fans was higher than that measured from floors, the count at the end of the growing-out period reached (59.75 ± 63.99 CFU ×106 /g from fans) and (17.07 ±21.81 CFU ×106 /g from the floor).
Table 1: Total Fungal Count (TFC) of poultry environment samples from different selected poultry houses.
|
Sampling |
Water lines and water sources (CFU×104 / 20 cm2) |
Total fungal count of dust (CFU×104 /gm) |
Cooling pads water ( CFU /ml) |
|||
|
Time |
Housing |
A |
B |
Fans |
Floor |
|
|
10th Day |
H1 |
20 |
12.5 |
6750 |
50 |
700 |
|
H2 |
16 |
7.5 |
137.5 |
40 |
50 |
|
|
Mean ± SD |
18 ± 2.82 |
10 ± 3.53 |
3443.75 ± 4675.74 |
45 ± 7.07 |
375 ± 459.61 |
|
|
30th Day |
H1 |
39 |
6 |
10500 |
3250 |
5000 |
|
H2 |
13 |
32.5 |
1450 |
165 |
260 |
|
|
Mean ± SD |
26 ± 18.38 |
19.25 ± 18.73 |
5975±6399.31 |
1707.5±2181.42 |
2630±3351.68 |
|
(A) indicate the entrance of water line system expressed by (CFU x 104/20 cm2); (B) indicates the average of two ends of water line system expressed by (CFU x 104/20 cm2). H1: indicated house number one; H2: indicated house number two.
Table 2: Total Fungal Count (TFC) of poultry environment samples after disinfection.
| Sampling |
H1 |
H2 |
Mean±SD | |
|
Water lines after disinfection (CFU /20 Cm2) |
Entrance of water line system |
2 x104 |
5x103 |
1.25 ±1.06 |
| Average of two ends of water line system |
4x102 |
1x104 |
0.52 ±0.67 | |
|
Dust of Fans and Floor After Disinfection (CFU /20 Cm2) |
Fans |
7x102 |
50 | 375±459.61 |
| Floor | 154 | 20 |
87 ±94.75 |
|
H1: indicated house number one; H2: indicated house number two.
Table 3: The frequency of fungal isolates in different poultry environment samples before and after disinfection.
| Fungal Isolates | Water Lines | Cooling Bad Water | Fans | Floor | Total numbers and positives numbers and percentage of occurrence | |||||||||||||
| House 1 | House 2 | House 1 | House 2 | House 1 | House 2 | House 1 | House 2 |
Before disinfection |
After disinfection |
|||||||||
| A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | |||
| Aspergillus niger | + | + | + | - | + | - | + | + | + | + | + | + | + | + | + | + | 8/8 (100%) | 6/8 (75%) |
| Aspergillus flavus | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | + | 8/8 (100%) | 7/8 (87.5%) |
| Aspergillus Fumigatus | + | + | + | + | + | + | + | - | + | - | - | - | + | - | + | + | 7/8 (87.5%) | 4/8 (50%) |
| Fusarium sp | + | - | - | - | + | - | + | + | - | - | - | - | - | - | - | - | 3/8 (37.5%) | 1/8 (12.5%) |
| Penicillium sp | + | - | + | - | - | - | + | - | + | + | + | + | + | + | - | - | 6l8 (75%) | 3/8 (37.5%) |
| Mucor | + | + | + | + | + | - | + | - | + | - | + | + | - | - | + | - | 7/8 (87.5%) | 3/8 (37.5%) |
| Dematiaceous | - | - | + | + | - | - | - | - | - | - | + | + | - | - | - | - | 2/8 (25%) |
2/8 (25%) |
(+): present (-): absent (A): before disinfection (B): after disinfection.
Total fungal count following disinfection
The results investigated the reduction in the count of fungi after disinfection (Table 2), it reached 1.25 ±1.06 CFU ×104 /20 cm2 in entrance of water line system and 0.52 ±0.68 CFU ×104 /20 cm2 in the ends of the water line system. Total fungal counts of fans and floors were (375.00 ±459.62 and 87.00 ±94.75 CFU /20 cm2), respectively.
Fungal isolation and identification
Different fungal isolates (Aspergillus niger, Aspergillus flavus, Aspergillus Fumigatus, Fusarium sp., Penicillium sp., mucor, and Dematiaceous sp.) were identified macroscopically and microscopically on a wet mount technique slide coated with lactophenol cotton blue dye and examined under the microscope with a 40x lens (Figures 2, 3 and 4).
Frequency of fungal isolation in different poultry sources before and after disinfection
Results revealed a reduction in percentage of the fungal isolates from waterlines, fans, and floor after disinfection, as documented in Table 3 and Figure 1. The isolation rate of Aspergillus niger has decreased from 100% to 75.0%, Aspergillus flavus from 100% to 87.5%, Aspergillus Fumigatus from 87.5% to 50.0%, Fusarium sp. from 37.5% to 12.5%, Penicillium sp. from 75.0% to 37.5%, and mucor from 87.5% to 37.5%.
Mold contamination is widespread in tropical areas with rapidly expanding poultry production. Fungal contamination plays a key role in the induction of diseases in poultry farms, as well as immune suppression, which promotes the spread of infectious diseases among flocks. Several studies have looked at the bacterial community composition of poultry houses, but there were few reports on mycological data. There is no published information on the isolation of fungal isolates from cooling pads. In addition to water line sampling, surface sampling is required for evaluating fungal contaminants, identification, and classification (Stetzenbach et al., 2004; Dumas et al., 2011).
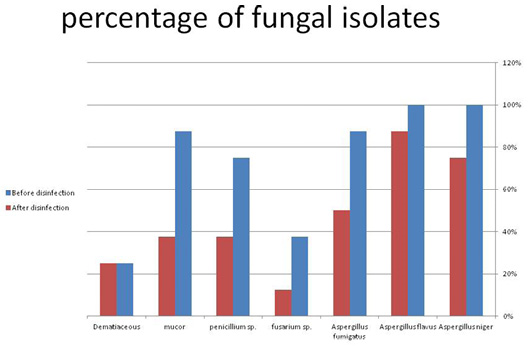
Figure 1: The occurrence of fungal isolates before and after disinfection. (A) before disinfection; (B) after disinfection.
In the current study, the prevalence of fungal contaminants was identified in different poultry house environment samples (water line, cooling pads’ water, and dust from fans and floors). Almost all the findings increased as the poultry gets older and heavier (Table 1). Our findings are consistent with those of (Maharjan, 2016) who found occasional increases in microbial levels of more than 4 log10 CFU/ml in drinking water during the flock grow-out period. The contamination by total fungal count in water lines reached the highest level in the entrance of water (18.00 ± 2.83 and 26.00 ± 18.38 CFU ×104 / 20 cm2) in house 1 and house 2, respectively, compared to the average TFC at two ends of water line system which recorded (10.00 ± 3.54 and 19.25 ± 18.74 CFU ×104 / 20 cm2), respectively in same houses. In such cases, the concentrations of fungi may decrease at the end of water lines due to dilution of water pressure as large amounts of water are daily consumed. Results from cooling pads water show high TFC at the end of rearing period reached to 26.30±33 ×102 CFU/ml compared to the first week 3.75±45 ×102 CFU/ml. These results illustrated the importance of performing routine water sanitation and line cleaning to solve much of the microbial problem in water systems (Watkins, 2006).
Since endothelial cells desquamation, as well as feed, skin, feces, and other sources, cause significant amounts of dust to be released into the atmosphere by chickens, the total fungal count (TFC) from dust collected from fans and floor (Table 1) increased toward the end of the fattening time (Takai et al., 1998). Because of the lower particle density and the ability of smaller particles to move long distances, bioaerosol formation occurs, resulting in longer distance transport (Millner, 2009). According to the findings, dust from fans has higher fungi loads than dust from the floor, and dust from the ground has lower fungi loads. (59.75±63.99, 17.07±21.81 CFU ×106 /g), respectively at the end of poultry rearing. Animal behavior, ambient temperature, humidity, airflow, animal stocking density, animal mass, species of bird, bird aged, the form of feed, eating phase, time of day, relative locations of dust sources, and the presence or absence of air cleaning technologies are just a few of the variables that affect fungal contamination in waste that has been around for a while (Ellen et al., 2000; Whyte, 2002). Occupational exposure levels for organic dust and endotoxins are routinely surpassed in various countries, putting workers at risk of negative health consequences (Burch et al., 2009; Wouters et al., 2006).
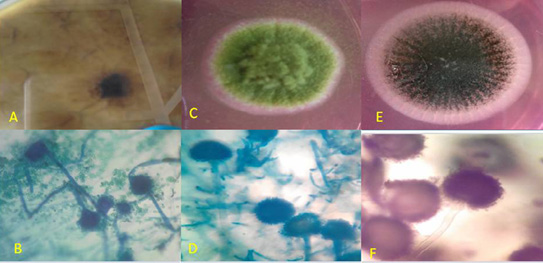
Figure 2: Macroscopic and microscopic by Lacto phenol cotton blue (LPCB) wet mount slide prepared from a micro-culture technique of Aspergillus fumigatus, Aspergillus flavus, and Aspergillus niger.
A- Macroscopic Observe of Aspergillus fumigatus: downy blue green with light margin B- Microscopic of A. fumigatus; Hyaline septated hyphae, short colorless to light green smooth conidiophores, round columnar conidialvesicle and finally uniseriate phialides.
c. Macroscopic Observe of Aspergillus flavus: downy yellow green with light margin.
d. Microscopic of A.flavus: Hyaline septated hyphae, colorless rough conidiophores, round radiate conidial vesicle and finally uniseriate phialides with sclerotia.
e. Macroscopic: Observe of aspergillus niger: slightly rough black; Reverse: white to yellow
f. Microscopic of A. niger: Hyaline septated hyphae, long colorless smooth conidiophores, globose brown to carbon black round radiate conidial vesicle and finally biseriate phialides chained columns of dark black spores.
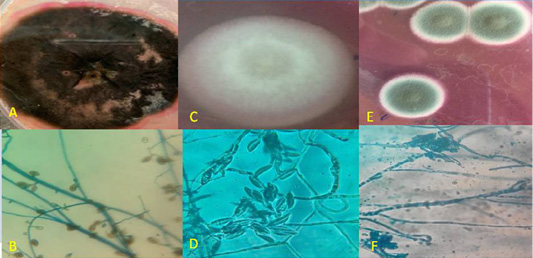
Figure 3: Macroscopic and microscopic by Lacto phenol cotton blue (LPCB) wet mount slide prepared from a micro-culture technique of Dematiaceous, Fusarium sp. and Penicillium sp.
a. Macroscopic of Dematiaceous: Observe leathery dark brown; Reverse is black. b. Microscopic of Dematiaceous septated hyphae with brown septa, brown colored conidiophores, and carry large sized brown club shaped conidia which are septated both longitudinally and transversely.
c. Macroscopic of Fusarium sp. Observe: cottony coral red; Reverse is white to pale yellow.
d. Microscopic of Fusarium Hyaline septated hyphae, single un branched conidiophores, banana shaped macroconidia.
e. Macroscopic of Penicillium sp. Observe: dense velvet felt shades of green; Reverse is white to yellow.
f. Microscopic: of penicillium Hyaline septated hyphae, long colorless smooth conidiophores,a brush like metulae, and carring single row of philides, finally ends with long chain of branched and unbranched rows of conidia.
Regarding macroscopic and microscopic detection of fungi, we isolated seven species: Aspergillus flavus and Aspergillus niger (100%), Aspergillus fumigatus and Mucor sp. (87.5%), Penicillium sp. (75.0%), Fusarium sp. (37.5%), and Dematiaceous isolated only from the water line and fans in house 2 (25.0%). According to (Zorman and Jeršek, 2008) mold, quantitative measurements would be underestimated if only traditional fungal measurement approaches, such as fungal culture, were used. Furthermore, in heavily polluted conditions, the presence of other organisms can be obscured by the presence of dominant rapidly developing fungi (Bartlett et al., 2004). Some of the filamentous fungi obtained in this study have been linked to human and animal infections (Ponikau et al., 1999; Araújo et al., 2003; Viegas et al., 2011). In Zagreb, (Rimac et al., 2010) found that species from the genera Penicillium sp., Fusarium sp., Aspergillus sp., Mucor sp., Rhyzopus sp., Scopulariopsi sp., Penicillium sp., and Fusarium sp., were the most common fungi. Other research in agricultural facilities in Ukraine and Poland found that these fungi were the most common genera (Tsapko et al., 2011). As a consequence, aspergillosis is a leading cause of illness and death in poultry, wreaking havoc on both the environment and the economy. It is also worth noting that Aspergillus fumigates, which has been found in poultry farm litter and in the air (Viegas et al., 2012), is one of the most common saprophytic fungi in the atmosphere and can cause severe or even fatal aspergillosis (Yao and Mainelis, 2007). Furthermore, Aspergillus flavus, released in the environment of a chicken farm, is a well-known source of harmful mycotoxins (aflatoxins). Mycotoxins are present in isolated genera such as Fusarium and Penicillium, in addition to this genus (Araújo et al., 2003).
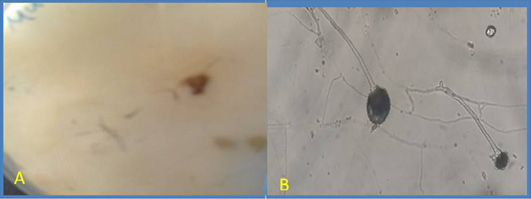
Figure 4: Macroscopic and microscopic of Mucor.
(A) Macroscopic: Observe: fluffy white; Reverse: pale yellow; (B)Microscopic: Lacto phenol cotton blue (LPCB) wet mount slide prepared from a micro-culture technique of Mucor suspected macroscopic colonies, examined under 40xobjective lens of STD-9 LED (Finelab®) light microscope. Hyaline aseptated hyphae, long colorless erect simple sporangiophores that forms a terminal globose multispored grey smooth fine walled sporangia, reminant of sporangial wall appear as a conspicuous collarette.
Waterborne infection control in broiler production requires disinfecting water and water sources, as well as managing microbiological issues related to water (Hamdullah et al., 2010). The TFC after disinfection is shown in Tables 2, 3, Figure 1, along with all the findings indicating a reduction in the number of fungi after disinfection. Disinfection was achieved using glutaraldehyde. Other studies have shown that Glutaraldehyde has a fungicidal activity against A. niger, A. terreus, M. racemosus, and R. nigricans at a 4.0 log reduction. Longer exposure times, up to 30 minutes, are needed (Akamatsu et al., 1997; Tortorano et al., 2005). According to (Howie et al., 2008) 2 percent glutaraldehyde causes a more than 4.0 log reduction of C. albicans on a PVC carrier surface in 1 minute, indicating high yeasticidal operation.
CONCLUSIONS AND RECOMMENDATIONS
According to our study, the prevalence of airborne and waterborne fungi in poultry housing varies depending on the surface. Seven fungal species were isolated; Aspergillus flavus, Aspergillus niger, Aspergillus fumigatus, Mucor sp., Penicillium sp., Fusarium sp. From water and dust samples, while Dematiaceous sp. was isolated only from the waterline and the fans in house 2. However, since fungal pollution affects the safety of the environment and drinking water in commercial broiler farms, effective disinfection procedures must be used. Total fungal count levels were found to be lower after disinfection. However, some of them survived after disinfection.
Novelty Statement
Few papers on mycological contamination; especially isolation of fungal isolates from cooling pads’ water. As a consequence, this research aimed to isolate and identify airborne and waterborne fungi from a variety of surfaces in two closed broiler chicken housing in the Giza government.
Author’s Contribution
Conceptualization, Hanan S Khalefa and S.T. Moubarak
; methodology, investigation, andresources validation, Aya Nasser Ibrahim, Hanan S Khalefa, and Hassan Aboul-Ella; formal analysis and data curation, S.T. Moubarak writing—original draft preparation, Writing review and final editing, Aya Nasser Ibrahim, Hanan S Khalefa, and S.T. Moubarak Visualization, Hanan S Khalefa.; supervision, S.T. Moubarak. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References






