Journal of Animal Health and Production
Research Article
Physico-Chemical Quality and Calorific Value of Buffen, Venison and Chevon
Fariya Sial1, Ghulam Shabir Barham1*, Atta Hussain Shah1, Gul Bahar Khaskheli1, Muneer Ahmed Jamali1, Paras Khatoon Siyal2
1Department of Animal Products Technology, Faculty of Animal Husbandry & Veterinary Sciences Sindh Agriculture University Tandojam, Pakistan; 2Department of Veterinary Parasitology, Faculty of Animal Husbandry & Veterinary Sciences Sindh Agriculture University Tandojam, Pakistan.
Abstract | Present study was carried out to analyze the physico-chemical quality including nutrient profile of buffalo (buffen), deer (venison) and goat (chevon) meat. Eighteen fresh meat samples (n=6 per meat type) were collected and analyzed for physical, chemical and nutritive attributes. The pH value (6.02±0.063), drip loss (3.81±0.047%) and cooking loss (36.92±0.277%) in chevon were observed higher (P<0.05) than the pH (5.91±0.039 and 5.72±0.046), drip loss (3.42±.010 and 2.89±0.178%) and cooking loss (32.51±0.301 27.79±0.348%) in buffen and venison, respectively. While, the water binding property of buffen (67.71±0.007%) found remarkably (P<0.05) higher compared to chevon and venison (63.58±0.139 and 54.30±0.850%). Reduced moisture (71.09±0.105%) with increased (P<0.05) protein and ash (22.98±0.040 and 3.16±0.102%) contents were observed in Venison than that of buffen (73.88±0.041, 20.03±0.059 and 1.84±0.060%) and chevon (76.01±0.020, 20.59±0.046 and 31.31±0.039%). However, fat and glycogen (4.01±0.043%; 22.71±0.339mg/g) were found high (P<0.05) in buffen against venison (2.54±0.033 and 21.80±0.299mg/g) and chevon (1.90±0.019 and 18.45±0.134mg/g), respectively. Furthermore, calorific values (122.71±0.567 and 121.85±0.365 Kcal/100g-1) of buffen and venison varied non- significantly to each other, but found relatively (P<0.05) higher than chevon (105.86±0.207 Kcal/100g-1). On the basis of current findings, it is concluded that the deer (venison) and buffalo meats (buffen) are more nutritious than goat (chevon) meat because of their elevated protein, fat and total mineral contents, which are vital for the growth and body requirement of consumers.
Keywords | Physico-Chemical attributes, Calorific value, Buffen, Venison and Chevon
Received | December 29, 2020; Accepted | January 19, 2021; Published | April 15, 2021
*Correspondence | Ghulam Shabir Barham, Department of Animal Products Technology, Faculty of Animal Husbandry & Veterinary Sciences Sindh Agriculture University Tandojam, Pakistan; Email: gsbarham@sau.edu.pk
Citation | Sial F, Barham GS, Shah AH, Khaskheli GB, Jamali MA, Siyal PK (2021). Physico-chemical quality and calorific value of buffen, venison and chevon. J. Anim. Health Prod. 9(2): 178-184.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.2.178.184
ISSN | 2308-2801
Copyright © 2021 Barham et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Worldwide, meat is the most fundamental component in the diet of consumers; physically and biologically it proved great impact on the individual health, economical system and development. It is an excellent food commodity having more protein, minerals; selenium, phosphorus, iron, zinc and B-complex and D Vitamins (calciferol). Due to higher nutritive food commodity, meat has complete nutritive profile in relation to whole calories to complete each day protein needs (da Silva et al., 2015). Animal origin protein sources have been obtained from domestic and wild animals (Arain et al., 2010). Per capita requirement of protein is 27grams, while people are simply capable to gain only 17grams of protein with shortage of 10grams, this gap of protein supply may possibly be fulfilled by the use of animal protein (meat) (Sohaib & Jamil, 2017). Sensorial attributes of meat i.e. juiciness, aroma, colour, tenderness and taste with its nutritional characters are influenced by genetics, livestock management practices, feeding, slaughtering and storage conditions of meat (Hocquette et al., 2012). Buffalo meat is scientifically termed as buffen or Carabeef; it is lean high in protein, minerals; iron, calcium, zinc, saturated lipids, vital amino acids and B complex vitamins. It is similar to red meat gained from additional sources in favor of its essential, dietary, plus organoleptic characteristics and trend of utilization of buffen in modern meat processing is increasing due to its good binding properties and lean quality (Tamburrano et al., 2019; Rey & Povea, 2012). Meat of wild animals is commonly known as game meat and meat of deer technically and scientifically called as venison. Both from cooking and cultural perspectives wild animal’s meat have significant importance for ornamental tradition and linking of animal production with country, which revolved the economy of region (Huerta-Leidenz et al., 2016). Six deer breeds have been documented in the mountainous, desert and irrigated areas of Pakistan, renowned as: Axis deer, Black buck, Indian Gazelle, golden deer, Barking deer and Himalayan Musk (Wild life of Pakistan, 2019-2020). Deer meat (Venison) contains high level of protein, low cholesterol lipids, minerals; zinc, sodium, phosphorus, selenium, calcium, magnesium, iron, copper, potassium and chromium, and valuable A, B, C, D vitamins (Gizejewska et al., 2016). Goat meat (chevon) is a vital resource of essential protein sub units, saturated fatty acids with intramuscular fat and minerals. It deemed as lean meat and its nutritional profile satisfy the consumer due to its palatable attributes compared to cattle meat (Horcada et al., 2012). Instead of having excellent source of vital nutrients of animal origin scarce work has been done before in Sindh, Pakistan. Therefore, current study was done to explore the physico-chemical quality including nutrients profile of buffalo, deer and goat meat (buffen, venison and chevon).
MATERIALS AND METHODS
Experimental Design
All the collected meat samples were replicated for the analysis, a total of six (6) number of buffalo and goat meat samples were purchased from market of Tandojam, and same number of deer meat (venison) samples were obtained from desert part of Tharparkar district of Sindh. Investigational tactic was used to assess the comparative physico-chemical quality and calorific value of buffalo, deer and goat meat samples at analytical laboratory of Department of Animal Products Technology, Faculty of Animal Husbandry and Veterinary Sciences, Sindh Agriculture University Tandojam.
pH value
In a glass beaker 10g of minced meat sample was mixed in 90ml of distilled water and an electrode of pH meter along with probe of temperature was inserted into the homogenized meat sample. Constant end result displayed on the pH meter was recorded as pH value of each meat sample.
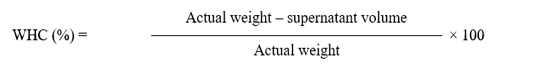
Water holding capacity
The scientific protocol invented by Wardlaw et al. (1973) was used to determine the water holding capacity of all meat samples. Briefly, 8 grams of each minced meat sample was placed in a centrifuge tube, later on each centrifuge tube was filled with 0.6 molar NaCl solution (12ml). After thoroughly mixing all the prepared samples were centrifuged in Backman centrifuge machine at 4ºC for 15minutes at 10,000 revolutions per minute and at the end of centrifugation, upper most liquid was poured in a measuring cylinder for calculation.
Cooking loss
A protocol of Kondaiah et al. (1985) was adopted for the analysis of cooking loss of all meat samples. 20g of meat sample was enclosed in a polyethylene bag and placed in the water bath adjusted to 80ºC (to provide inner hotness of about 72ºC) for 1 hour. Fluid expelled out due to cooking pressure was poured out and the cooked mass with the help of filter paper was properly dried off and then re-weighted. The formula used for cooking loss of meat is given below.
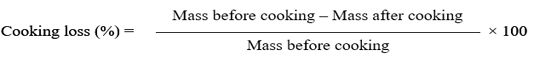
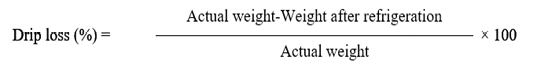
Drip loss
A protocol as illustrated by Sen et al. (2004) was used to analyze the drip loss of all meat samples. 50 grams of meat samples was packed in a polyethylene bag, then kept for 24 hours in refrigerator at 4ºC with seal coat, all the meat samples were taken out from refrigerator after 24 hours, then with the help of filter paper meat sample was soaked and dried off, at the end samples were reweighted and drip loss was calculated by formula given below.
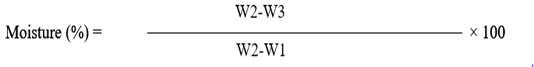
Moisture content
The scientific approach as mentioned in AOAC (2005) was followed for analysis of moisture content in all of meat samples. In a pre-weighted dehydrated aluminum dish, 5grams of meat sample was taken and placed into a hot air oven (at 100ᴼC) for 4 hours, then dehydrated meat sample was kept for one hour in a desiccator, lastly, the aluminum dish having dried meat sample was re-weighed, formula given below was used for moisture content.
Protein content
2g meat was assimilated with 0.35g of copper sulphate, 7g of potassium sulphate along with 30ml of sulfuric acid as an oxidizing agent. With 250ml of distilled water completely digested sample was diluted, then in the presence of 40 % NaOH solution, 5ml of diluted sample was distillated and the vapors (ammonia) were trapped over 5ml of boric acid (2%) having bromocresol green as an indicator for 4 minutes by using Kjeldhal distillation (AOAC, 2005). Lastly, sample was titrated with 0.1NHCl to determine Nitrogen and nitrogen content was computed by using formula as given below.
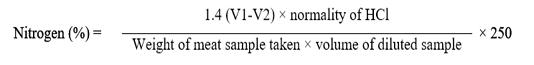
Protein content was computed by converting the obtained nitrogen into protein by using conversion factor (CF) i.e. 6.25.
Protein percentage = N% × Conversion factor (CF)
Fat content
2g of dried meat was taken in grease free thimble, placed in Soxhlet extraction unit, while dehydrated and pre-weighted distillation flask containing petroleum ether (150ml) was assembled with condenser and Soxhlet Extractor, and solvent was boiled by placing on electric heater, distillation flask was removed to cool down and dried in oven and re-weighted after approximately 6 hours of extraction (AOAC, 2005). Fat content of meat was calculated by following formula.

Glycogen content
A process invented by Kemp et al. (1953) was used to determine the glycogen content of meat. 0.2g of meat sample was placed with 5ml of de-proteinizing solution in Backmen centrifuge tube. In water bath till 15 minutes tube containing samples were boiled, and cooled with running water. Then at 4°C for 5 minutes at 3000rpm samples were centrifuged. After that 1ml of clear supernatant in test tube and 3ml of H2SO4 was added, vigorously mixed and heated for six minutes. At wave length of 520μm the strength of color was calculated with the help of spectrophotometer. Results which showed on the screen of spectrophotometer were noted as glycogen level.
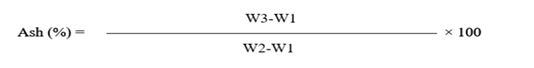
Ash content
By using of Gravimetric method as mentioned in AOAC (2005) evaluated the ash content of all meat samples. Taken 5g of meat in empty pre-weighed crucible dish, then in a muffle furnace for 5 hours at 550°C crucible having meat was moved to ignite sample, lastly ashed meat sample transferred in desiccators till 1hour the dish was weighed again. By using formula given below ash content of meat was calculated.
Calorific/Nutritive value
Calorific values of buffen, venison and chevon meat samples were calculated by using energy conversion factors of major components as reported by Johnson et al. (1995). Like 4 for protein, 9 for fat and also 4 for carbohydrates.
Kcal (per 100g) = [(% protein) (4)] + [(% fat) (9)] + (% Carbohydrates) (4)]
Data analysis
By using Excel Microsoft program, primarily data so obtained was gathered, tabulated and analyzed by applying statistical tools; analysis of variance (ANOVA) by using computer software, Student Edition of Statistix (SXW), Version 8.1. Significant variation subsisted between the averages; further averages were computed by LSD test at the level of 5% probability.
RESULT AND DISCUSSION
Physical Characteristics
Results of current investigation described in Table-1 showed that the ultimate pH values was noted relatively (P<0.05) higher (6.09±0.063) in fresh goat meat (chevon) contrast to buffalo (5.91±0.030) and deer meat (5.71±0.045). Contrast to current results, Fazlani et al. (2019) recorded 6.92 pH values in goat meat; Arain et al. (2010) recorded pH range (6.20 to 6.40) in the chevon of dissimilar age groups of goat; comparatively lower pH (6.00) of buffalo meat was recorded by Naveena et al. (2011), similarly Kandeepan et al. (2009) and Okuskhanova et al. (2017) noted 5.73 final pH in buffalo meat compared to 5.85 pH of venison. In the same way, a group of scientists described 5.5 to 5.90 pH value of deer meat, which parallels to current results (Marcio et al., 2019; Abellan et al., 2018). Mean water hold
Table 1: Physical characteristics of buffen, venison and chevon.
|
Physical characteristics of meat |
Meat of different animals* |
LSD (0.05) |
SE±
|
||
| Buffen | Venison | Chevon | |||
| pH |
5.91±0.030b |
5.72±0.046c |
6.09±0.060a |
0.1784 | 0.0801 |
| Water holding Capacity (%) |
67.69±0.006a |
54.30±0.850c |
63.58±0.130b |
1.5566 | 0.6986 |
| Cooking loss (%) |
32.51±0.300b |
27.79±0.340c |
36.92±0.270a |
0.9600 | 0.4308 |
| Drip loss (%) |
3.42±0.011b |
2.90±0.178c |
3.81±0.047a |
0.3472 |
0.1558 |
Superscripts with different letters in same row varied significantly (P< 0.05) with each other.
* n=6 for each type of meat
Table 2: Chemical characteristics and calorific/nutritive value of buffen, venison and chevon.
|
Chemical characteristics of meat |
Meat of different animals* |
LSD (0.05) |
SE±
|
||
| Buffen | Venison | Chevon | |||
| Moisture (%) |
73.88±0.040b |
71.41±0.105c |
76.10±0.020a |
0.1900 | 0.0853 |
| Protein (%) |
20.31±0.059c |
22.98±0.040a |
20.60±0.046b |
0.1287 | 0.0578 |
| Fat (%) |
4.18±0.043a |
2.54±0.033b |
1.90± 0.019c |
0.1103 | 0.0495 |
| Ash (%) |
1.98±0.059b |
3.47±0.102a |
1.31± 0.038c |
0.2650 | 0.1190 |
| Glycogen (mg/g) |
22.71±0.339a |
21.80±0.299a |
18.45±0.134b |
0.9142 | 0.4103 |
| Calorific/Nutritive value (Kcal/100g) |
122.75±0.567a |
122.03±0.364a |
106.00±0.207b |
1.1082 |
0.4974 |
Superscripts with different letters in same row varied significantly (P< 0.05) with each other.
* n=6 for each type of meat
ing capacity (WHC) was noticed high (P<0.05) in buffalo meat (67.69±0.006%) than that of chevon (63.58±0.139%) and venison (54.30±0.859%) samples of meat (Table-1), parallel to present results the higher levels of water holding capacity (69.00%) in buffalo and deer meat (65.82%) was recorded by different authors compared to water binding activity (63%) of goat meat (Okuskhanova et al., 2017; Arain et al., 2010). It is note of interest that water holding activity of meat positively correlated with pH; muscle with high pH could display a better movement of water withholding due to strong binding activity of water with protein of meat (Cawthorn et al., 2018). Results highlighted in Table-1 revealed statistical (P<0.05) disparity in cooking loss noted comparatively higher in chevon (36.92±0.277%), medium in buffen (32.51±0.301%) and lower in venison (27.79±0.348%). Alike to present results, in goat meat 47.30% cooking loss was reported by Antonius et al. (2020), 28.02 to 38.46% in chevon was also reported by some other scientists (Fazlani et al., 2019; Rodriguez et al., 2011), where modest losses during cooking in buffen was recorded by Mello et al. (2017), though minute losses of fluids during cooking were noticed by Purchas et al. (2010) in the meat of domesticated red deer. Comparatively (P<0.05) higher drip loss in chevon (3.81±0.047%) compare to buffen (3.42±0.011%) and venison (2.90±0.178%) was noted in current study (Table-1). In accordance, 2.10 to 5.70%, purging of fluids in goat meat have been cited by Fazlani et al. (2019) and Arain et al. (2010), moderate drip in buffen was documented by Naveena et al. (2011), and 2.10% of drip was noticed in deer meat by Vita et al. (2020) and Ludwiczak et al. (2016). Not surprisingly, ultimate muscle pH is a main issue that influences different meat quality characteristics, as well as the water maintenance capacity during cooking, the loss of fluids, exclusion of water, palatability attributes and physical appearance of meat (da Silva et al., 2015).
Chemical Characteristics of Buffen, Venison and Chevon
The average moisture (76.10±0.02%) in chevon noted appreciably (P<0.05) higher, modest in buffen (73.88±0.040%) and lesser in venison (71.41±0.105%) meat samples (Table-2). In accordance, Arain et al. (2010) and Shija et al. (2013) reported 73 to 78.30% moisture content in chevon, Awan et al. (2014) noted 75 % level of moisture in buffalo meat, whereas in the meat of deer 74 to75.3% of moisture was noticed by Dahlan et al. (2008). It is interesting to note that a difference in postmortem final meat pH is a major factor which influence on water content and additional quality characteristics of meat (da Silva et al., 2015). Protein level evaluated reasonably (P<0.05) more in venison (22.98±0.040%) compared with chevon (20.60±0.046%) and buffen (20.31±0.059%) meat samples (Table-2). Contrast to present findings Arain et al. (2010) and Adim et al. (2008) reported 19-21% protein in goat meat; Kim et al. (2006) reported 22% crude protein in buffen. Similar to current results a panel of researchers cited 23.67% protein in venison (Ivanovic et al., 2013, Vita et al. 2020; Amici et al., 2015), respectively. Different researchers have strong recommendations about the higher concentrations of protein and fat in buffen, beef and venison followed by goat meat had good value of protein and lowest level of fat (Okuskhanova et al., 2017). Table-2 indicates that level of fat was recorded comparatively (P<0.05) more in buffen (4.18±0.043%), moderate in venison (2.54±0.033%) and lower in chevon (1.90± 0.019%). Similar with present findings, Awan et al. (2014) noted 4-6% fat in buffen; Kim et al. (2006) and Vita et al. (2020) reported 2-2.5% fat in venison; Arain et al. (2010) rerecorded 2-2.5% fat in the chevon. However in spite of having low fat in deer compared to buffen and beef, venison possessed organoleptic and palatability characteristics respect to all types of meats (Bures et al., 2015), though it is also a fact that buffalo and goat meat is attributed with higher concentration of fat with lower concentration of saturated fatty acids than venison (Okuskhanova et al., 2017). In the current study comparatively (P<0.05) higher ash (3.47±0.102%) content was evaluated in venison followed by buffen (1.98±0.059%) contrast to ash content of chevon (1.31± 0.038%) samples (Table-2). Alike, in venison 1.5-2.21% ash was noted by Okuskhanova et al. (2017) compared to 1.15% in buffen and 1.4-1.8% of ash in chevon was cited by Arain et al. (2010), respectively. In addition, contrary to current results, Alamin et al. (2014) found 0.47% of ash content in buffen and 0.43% in chevon. Glycogen level in buffen (22.71±0.339mg/g) noticed appreciably (P<0.05) higher, moderate in venison (21.80±0.299mg/g) and lower in chevon (18.45±0.134mg/g) samples (Table-2). In accordance, Fazlani et al. (2019) reported 14.74 to 19.21mg/g of glycogen in chevon, the level of glycogen in buffalo meat noted in the current research is comparable with the results reported by Warriss (2000). He reported 10-20mg/g of glycogen and 5.5-6.0 ultimate pH values in good quality meat. In the present findings better concentrations of glycogen in red deer meat might be due to long muscular activity by distance covered for the searching of fodder by the wild animals in their environment compared to domestic fattened animals fed on stall feeding (Wiklund et al., 2008).
Calorific/nutritive Value of Buffen, Venison and Chevon
Results depicted in Table-2 revealed that calorific/nutritive value was estimated significantly (P<0.05) more in buffen and venison (122.71±0.567 and 122.03±0.364kcal/100g) than chevon (106.00±0.207kcal/100g). Usually, buffen contained higher concentration of intra muscular fat compared to venison and chevon, having low level of fat that might be the reason behind the higher nutritive values of buffen than deer and goat meat (Rail, 2018). Naveena et al. (2011) reported 122 calories in buffen contrast to beef due to lower quantity of fat, cholesterol and saturated fatty acids. The nutritional value of meat is directly related with the macro- and micro-nutrients like essential amino acids, minerals, protein, polyunsaturated fats, fat and B complex vitamins (Ossipova, 2013).
CONCLUSIONS
In conclusion, among physical attributes, pH, cooking loss and drip loss were found higher in goat meat (chevon), whereas water holding capacity was noticed higher in buffen and deer meat (venison) followed by goat meat (chevon). Furthermore, the deer (venison) and buffalo meat (buffen) were found to be rich in nutrients, which are quite essential for the nourishment of living beings in contrast to goat (chevon) meat.
ACKNOWLEDGENTS
Authors want to thank the staff of Department of Animal Products Technology, Faculty of Animal Husbandry and Veterinary Sciences, Sindh Agriculture University Tandojam.
CONFLICT OF INTEREST
Authors have no any conflict of interest.
authors contribution
Ghulam Shabir Barham, and Atta Hussain Shah, designed the research experiment for M.Phil degree research.
Gul Bahar Khaskheli and Muneer Ahmed formulated the data of research and conducted the statistical analysis.
Fariya Sial conducted the research experiment. and Paras Siyal helped her during research trial.
REFFERENCES






