Journal of Animal Health and Production
Research Article
Carcass Characteristics and Meat Quality of Broiler Chicken Fed Diets Supplemented with Pausynistalia yohimbe and Larvicide (Cyromazine)
Olusola Victor Obajuluwa1*, Kehinde Atinuke Sanwo2, Lawrence Tokunbo Egbeyale2, Adeboye Olusesan Fafiolu3
1Livestock Science and Sustainable Environment, Centre of Excellence in Agricultural Development and Sustainable Environment, Federal University of Agriculture, Abeokuta, Nigeria; 2Department of Animal Production and Health, College of Animal Science and Livestock Production, Federal University of Agriculture, Abeokuta, Nigeria; 3Department of Animal Nutrition, College of Animal Science and Livestock Production, Federal University of Agriculture, Abeokuta, Nigeria.
Abstract | Larvicidal herbs may help improve meat qualities and reduce residual synthetic chemicals in poultry meat. A total of 225 floor raised broiler chickens were equally divided into five treatment groups and fed with either basal (control) diet, basal diet supplemented with a commercial larvicide (Cyromazine, 5mg/Kg), or three levels of a larvicidal herb Yohimbe (Pausynistalia yohimbe) at (60, 120, and 180mg/Kg) diets. Two chickens per replicate from each of the five treatment groups were slaughtered to evaluate the carcass traits and meat quality. Results showed that Yohimbe has no significant (p>0.05) effects on the weights and cut parts of the chickens. However, there were significant (p<0.05) increase in relative weights of heart and kidney with 180mg/Kg Yohimbe supplement while the spleen relative weight was reduced. Although, sensory qualities such as: juiciness, meaty flavour and overall acceptability were improved (p<0.05) with 180mg/Kg Yohimbe supplementation, crude fibre in meat was reduced (p<0.05) with 60mg/Kg Yohimbe supplement, while lipid profile was unaffected by Yohimbe supplementation. The research concluded that supplementation of broiler diet with 180mg/Kg Yohimbe improves sensory qualities but Yohimbe has casual effect on carcass traits and proximate composition.
Keywords | Broiler chicken, Larvicide, Pausynistalia yohimbe, Carcass traits, Meat quality
Received | July 10, 2020; Accepted | November 27, 2020; Published | December 01, 2020
*Correspondence | Olusola Victor Obajuluwa, Livestock Science and Sustainable Environment, Centre of Excellence in Agricultural Development and Sustainable Environment, Federal University of Agriculture, Abeokuta, Nigeria; Email: olusolaobajuluwa@gmail.com
Citation | Obajuluwa OV, Sanwo KA, Egbeyale LT, Fafiolu AO (2021). Carcass characteristics and meat quality of broiler chicken fed diets supplemented with Pausynistalia yohimbe and larvicide (Cyromazine). J. Anim. Health Prod. 9(1): 40-46.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.1.40.46
ISSN (Online) | 2308-2801
Copyright © 2021 Obajuluwa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Commercial poultry production is one of the major suppliers of animal protein. Broiler chicken as a component of poultry production supplies the market with white meat (Harriet et al., 2019). This type of meat is more preferred because it poses lesser health risk to consumers as well as it has lesser carbon footprint. Zapata and Carpio (2014) predicts demand for poultry meat is expected to rise globally from 118 million metric ton in 2017 to more than 131 million metric tons by 2026. In spite of the good fate of poultry production, the problem of waste management may be on the rise as a result of the increase in its intensity of production. Poultry wastes such as: used litter, manure, and dead chicken are medium in which fly-larvae grows and mature into housefly (Axtell, 1986).
In other to prevent the growth of housefly in the poultry house, cyromazine was included into poultry diet as an insect growth regulator (Vazirianzadeh et al., 2007). The inclusion of cyromazine (N- cyclopropyl- 1, 3, 5-triazine- 2, 4, 6- triamine) into chicken diet has occasioned unexpected challenges. Cyromazine and its metabolite melamine may induce health challenges to poultry consumers as it was found in poultry carcass after slaughtering (Bao et al., 2011). In addition, the presence of cyromazine in poultry waste contributes to environmental challenges occasioned by livestock production as it is found in soil water and contaminates water bodies around commercial farms (Ruicheng et al., 2011).
Herbs or herbal product with larvicidal properties may help curtail the threat poses to consumer, poultry and environmental health. Pausinystalia yohimbe (commonly called Yohimbe) is a tree plant which has the natural compound Yohimbine as one of its constituents. Yohimbine, an indole alkaloid was reported by Soon and Young-Joon (2017) to have larvicidal properties. Anonymous (2016) also recount that commercial Yohimbine is being taken by body builders to improve muscle mass, improve body weight gain and increase testosterone production in human. In addition, lypolitic properties of Yohimbine were observed by Berlan et al. (1991) who reported Yohimbine has fat reducing effect when ingested by obese and non-obese women. This lypolitic effect of Yohimbe may help in the rearing of lean meat in livestock production. Furthermore, the trial which involved the supply of Yohimbine to broiler chicken as carried out by Metin and Ahmet (2016) using 60 ppm and 120 ppm of the compound, shows an increase in protein accretion and reduction in the carcass fat content without a decline in the final weights and growth performance of broiler chicken. Therefore, this study intends to verify the effect of Yohimbe bark on carcass characteristics and some meat qualities of chicken.
MATERIALS AND METHODS
Ethical permit
The guidelines as approved by the project review committee of the College of Animal Science, Federal University of Agriculture, Abeokuta, Ogun state, Nigeria were adhered in the experimental practices adopted in this study.
Experimental setup
A total of 250 days old broiler chicks were brooded for two weeks after which they were raised for 6 weeks on the experimental diets. At two (2) weeks of age, 225 chicks were selected based on their weights and were assigned to the five (5) treatment groups with the average weights of each treatment groups equalized. Each treatment groups contain a total of 45 chickens which were further divided into triplicates of 15 birds. Clean water and feed were supplied ad-libitum throughout the period of the feeding trial. Medication and Vaccines which includes: coccidiostat, Infectious Bursal Disease and Newcastle disease vaccines were administered. Also, after brooding, chickens were kept at room temperature and were raised in a deep litter house. Starter mash was supplied when chickens were 3-4 weeks and finisher mash was supplied at 5-8 weeks old respectively. After the feeding trial, two (2) chickens from each of the three replicates in the five treatments were chosen by their live weights. The two selected chickens had live weights close to the average weight of the replicate groups. The chickens were slaughtered with their cut parts weighed and harvested for further analysis.
Experimental diets
Table 1 shows the composition of the basal diets used in this study, while the respective supplements fed to the chickens of various groups before slaughtering are shown below:
Control group = Basal diet without additives
Larvicide group = Basal diet with 5mg/Kg Larvicide (Cyromazine)
60mg Yohimbe group = Basal diet with 60mg/Kg of P. yohimbe bark
120mg Yohimbe group = Basal diet with 120mg/Kg of P. yohimbe bark
180mg Yohimbe group = Basal diet with 180mg/kg of P. yohimbe bark
Carcass evaluation
Two (2) chickens (of average weights) per replicate in each treatment was selected, fasted for 12hrs and slaughtered. After evisceration, the percentage dressed weights were recorded. The weight of the cut parts (head, shank, thigh, breast, neck, bark, wing and drumstick), organs (heart, kidneys, lungs, livers, gizzard, spleen, and caecum) and offal (intestine) were determined using a sensitive scale and expressed as percentage of the live weight.
The live weights of the selected birds were recorded using the weighing scale while alive at the end of 6 weeks of experiment. The birds were slit through the neck, allowed to bleed for two minutes and scalded in hot water at 65oC. Carcass feathers were plucked and carcass weights recorded. Carcasses were split and the viscera (internal organs) were removed. The weight of the carcass without the visceral was recorded as eviscerated weight of the birds. The dressed weight was calculated as under:
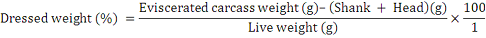
The organs (kidney, heart, spleen, liver and gizzard) weight were taken using the sensitive scale, and expressed as a percentage of the final live weight of the birds.
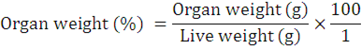
Fat around the cloacae, bursa of Fabricius, proventriculus, and muscles adjacent to the abdomen were considered as abdominal fat deposit (Cahaner et al., 1985). The thighs of each of the slaughtered chickens from the respective treatment groups were deboned by neatly separating the meat from the bone using clean razor. The skin was not separated from the meat but were weighed together. The respective weights of the meat and bone were recorded as described by Heyse and Marion (1973). Meat and bone weights were expressed as percentage of the cut part as reported by Metin and Ahmet (2016).
Table 1: Gross Composition of the experimental diet at the starter and finisher phases.
| Ingredients | Starter phase (%) | Finisher phase (%) |
| Maize | 52.50 | 55.00 |
| Wheat bran | 4.10 | 9.10 |
| Soybean meal | 18.50 | 14.00 |
| Groundnut cake | 16.50 | 15.00 |
| Fish meal | 2.50 | 1.00 |
| Bone meal | 3.00 | 3.00 |
| Limestone | 2.00 | 2.00 |
| Salt (NaCl) | 0.25 | 0.25 |
| Vit/Min Premix* | 0.25 | 0.25 |
| Methionine | 0.20 | 0.20 |
|
Lysine Total Calculated analysis(%) |
0.20 100.00 |
0.20 100.00 |
| Crude Protein | 22.29 | 20.13 |
| Calculated Metabolizable Energy (Kcal/Kg) | 3028.33 | 2864.77 |
| Ether extract | 4.53 | 4.00 |
|
Crude fibre Calcium Phosphorus |
3.40 2.00 0.93 |
3.54 1.94 0.67 |
*Premix composition per kg diet: Vit A: 400,000.00 IU, Vit D3: 800,000.00 IU, Vit E: 9,200.00mg, Vit k: 800.00mg, Vit B1: 1000.00mg, Vit B6: 500.00mg, Vit B12: 25.00mg, Niacin: 6000.00mg, Pantothenic acid: 2000.00mg, Folic acid: 200.00mg, Biotin: 8mg, Mn: 300,000.00g, Zn: 20,000.00g, Cobalt: 80.00mg, I: 40.00mg, Choline: 80,000.00g, Antioxidants: 800.00mg.
Sensory evaluation
A part of the breast meat from the slaughtered chickens were selected for sensory evaluation. Each of the breast samples were sealed in separate nylon bags to prevent the entry of water during cooking. The breast meat was cooked for 30 minutes at 70 oC in a laboratory water bath. Sensory evaluation was conducted immediately after cooking with ten panellists whom were selected for the assessment procedure. They were instructed to evaluate samples both by visual appraisal and chewing of samples from each treatment one after another in a random fashion. Samples were scored for colour, flavour, texture, juiciness and tenderness. Bottled water was served to the panellists to rinse their mouths after scoring each sample to reduce carryover effects. The panellists scored each sample on a nine point hedonic scale (9 = like extremely, 8 = like very much, 7 = like moderately, 6 = like slightly, 5 = neither like nor dislike, 4 = dislike slightly, 3 = dislike moderately, 2 = dislike very much and 1 = dislike extremely) (Sanwo et al., 2011).
Meat proximate composition
A part of the breast meat from each of the slaughtered chickens were taken for proximate analysis. Proximate composition which includes: moisture, crude protein (CP), ether extract (EE), ash and carbohydrate content of meat samples were determined by method described by AOAC (2005) using subcomponent 950.46 (39.1.02), 992.15 (39.1.16), 960.39 (39.1.05), 942.05, while utilizable carbohydrate was calculated by difference.
Meat pH
Approximately, 10g of meat samples from the breast muscle were weighed and placed in a clean sauce pan. The pH meter was switched on and allowed to stabilize for a period of five (5) minutes. The pH was standardized with buffer solution pH4, pH7, pH9 to ensure the sensitivity and accuracy of the meter. This was achieved by dipping the electrode of the meter into the respective buffer solution with thorough rinsing after each dipping. The pH was recorded by placing the same electrode of the hand held pH meter (Model PH-108A) deeply into the meat after rinsing the pH meter /apparatus in distilled water (Kim et al., 2008).
Determination of meat lipid profile
Composite paste from the breast muscle was prepared with a known amount of chloroform and methanol mixture 1:1 (v/v). The resulting paste solvent mixture were filtered and rinsed with an additional volume of the combined homogenate and allowed to stand for 5mins. The filtered homogenate was equilibrated to remove non-lipid material; 2% (0.32M) W/V KCl solution was added to the aqueous layer as described by (Folch et al., 1957) method. The filtrate was centrifuged and lipid extract was decanted. The extract was then made up to a final volume by adding chloroform. The decanted mixture to be obtained was used for the determination of cholesterol, triglyceride, high and low density lipoprotein respectively using commercial kits.
Statistical analysis
Data collected were subjected to Analysis of Variance (ANOVA) using Completely Randomized Design. The significant means were separated using Duncan’s Multiple Range Test (DMRT) at 5% probability level as contained in SAS (2010). The following equation was adopted as experimental model:
Yij= μ + Ti + Ɛij
Where:
Yij, Observed value of the dependent variable; μ, Population mean; Ti, Effects of graded levels of supplemented Yohimbe (0, Larvicide, 60, 120 and 180mg/Kg); Ɛij, Random residual error.
RESULTS
Effect of supplemented diets on carcass characteristics of broiler chicken
Table 2 exhibits effect of supplemented diets on carcass characteristics of broiler chicken. There were no significant differences (P>0.05) in the Live weights, bled weights, plucked weights, eviscerated weights, and dressed weights. Also, the cut part which includes: thigh, drumstick, back, breast, wings and abdominal fat percentages were insignificantly different (P>0.05).
Effect of supplemented diets on the internal organs of broiler chicken
Table 3 shows effect of supplemented diets on the internal organs of broiler chicken. Chickens fed Basal diet + Larvicide had significantly lower (P<0.05) relative heart and kidney weights. Spleen relative weight was significantly reduced (P<0.05) in chicken fed Basal diet + 180mg Yohimbe, while chicken fed Basal diet only had Proventiculus, duodenum, and Ileum + Jejunum with significantly reduced relative weights. Pancreas relative weight was significantly increased (P<0.05) in chicken fed Basal diet + 60mg Yohimbe.
Effect of supplemented diets on meat to bone ratio of broiler chicken
Table 4 showing effects of supplemental diets on meat to bone ratio of broiler chicken. Bone rate was significantly decrased (P<0.05) in chicken fed Basal diet + Larvicide, while Meat yield was significantly decreased (P<0.05) in chicken fed Basal diet only.
Table 2: Effect of supplemented diets on carcass characteristics of broiler chicken.
| Parameters | Control | Larvicide | 60mg Yohimbe | 120mg Yohimbe | 180mg Yohimbe | SEM |
| Live weight (g) | 2031.33 | 1983.33 | 1850.00 | 2000.00 | 1808.33 | 37.412 |
| Bled weight (%) | 96.04 | 94.27 | 95.51 | 94.98 | 94.94 | 0.307 |
| Plucked weight (%) | 93.13 | 89.08 | 90.52 | 91.48 | 91.40 | 0.740 |
| Eviscerated weight (%) | 79.03 | 77.03 | 79.79 | 77.83 | 78.29 | 0.551 |
| Dressed weight (%) | 71.60 | 70.96 | 71.72 | 71.12 | 70.87 | 0.577 |
| Thigh (%) | 11.31 | 11.21 | 11.98 | 11.91 | 11.76 | 0.150 |
| Drumstick (%) | 10.34 | 10.26 | 11.20 | 11.06 | 10.74 | 0.178 |
| Back (%) | 13.95 | 12.97 | 13.55 | 12.56 | 13.37 | 0.285 |
| Breast (%) | 21.66 | 22.20 | 20.84 | 21.02 | 21.38 | 0.370 |
| Wings (%) | 8.12 | 7.94 | 8.60 | 8.05 | 8.21 | 0.114 |
| Abdominal fat (%) | 1.65 | 2.07 | 1.93 | 1.59 | 1.55 | 0.097 |
SEM: Standard Error of Mean.
Table 3: Effect of supplemented diets on the internal organs of broiler chicken.
| Parameters | Control | Larvicide | 60mg Yohimbe | 120mg Yohimbe | 180mg Yohimbe | SEM |
| Heart (%) |
0.48 a |
0.40 b |
0.47 a |
0.54 a |
0.51a |
0.014 |
| Spleen (%) |
0.16 ab |
0.15 ab |
0.18 a |
0.19a |
0.11 b |
0.009 |
| Kidney (%) |
0.46 bc |
0.41 c |
0.57 ab |
0.58 ab |
0.59a |
0.024 |
| Liver (%) | 2.23 | 2.00 | 2.20 | 2.20 | 2.37 | 0.063 |
| Crop (%) | 052 | 0.52 | 0.58 | 0.55 | 0.59 | 0.016 |
| Proventiculus (%) |
0.42 b |
0.54a |
0.54 a |
0.51 ab |
0.50 ab |
0.017 |
| Pancreas (%) |
0.27 ab |
0.28 ab |
0.38a |
0.23 b |
0.32 ab |
0.019 |
| Gizzard (%) | 3.02 | 2.81 | 3.00 | 2.66 | 2.92 | 0.078 |
| Duodenum (%) |
1.11 b |
1.61 ab |
2.00 a |
1.82 a |
2.24a |
0.131 |
| Ileum + Jejunum (%) |
1.80 b |
2.71 ab |
3.74 a |
3.59 a |
3.61a |
0.241 |
| Caecum (%) | 2.55 | 2.10 | 2.64 | 2.01 | 2.23 | 0.113 |
| Duodenum length (cm) | 45.66 | 78.50 | 59.16 | 67.83 | 77.83 | 5.228 |
| Ileum + jejunum (cm) | 75.16 | 104.16 | 107.00 | 128.83 | 120.00 | 7.133 |
| Gizzard width (cm) | 4.66 | 6.58 | 11.75 | 4.41 | 4.25 | 1.370 |
| Gizzard length (cm) | 5.83 | 7.10 | 5.75 | 5.25 | 5.41 | 0.336 |
a,b,c Means in a row with different superscripts differ significantly (P<0.05). SEM: Standard Error of Mean.
Table 4: Effect of supplemented diets on meat to bone ratio of broiler chicken.
| Parameters | Control | Larvicide | 60mg Yohimbe | 120mg Yohimbe | 180mg Yohimbe | SEM |
| Bones (%) |
23.83a |
18.61 b |
20.05 ab |
21.04 ab |
19.48 ab |
0.724 |
| Meat yield (%) |
76.16 b |
81.38a |
79.94 ab |
78.95 ab |
80.51 ab |
0.724 |
a, b Means in a row with different superscripts differ significantly (P<0.05). SEM: Standard Error of Mean.
Table 5: Effect of supplemented diets on sensory scores of meat from broiler chicken.
| Parameters | Control | Larvicide | 60mg Yohimbe | 120mg Yohimbe | 180mg Yohimbe | SEM |
| Colour | 7.13 | 6.60 | 6.80 | 7.00 | 7.80 | 0.232 |
| Juiciness |
6.40 bc |
7.20 ab |
6.93 ab |
5.93 c |
7.46a |
0.179 |
| Meaty Flavour |
6.40 bc |
6.53 bc |
6.20 c |
7.20 ab |
7.60a |
0.174 |
| Tenderness |
7.40 a |
6.46 a |
6.40 a |
4.16 b |
7.20a |
0.386 |
| Saltiness | 5.00 | 4.53 | 4.60 | 4.20 | 4.80 | 0.170 |
| Overall flavour | 6.53 | 6.33 | 6.00 | 6.46 | 6.86 | 0.128 |
| Overall acceptability |
6.53 ab |
6.60 ab |
6.20 b |
6.46 ab |
7.33a |
0.156 |
a, b, c Means in a row with different superscripts differ significantly (P<0.05). SEM: Standard Error of Mean.
Table 6: Meat Proximate composition and pH value of chicken fed supplemented Yohimbe.
| Parameters | Control | Larvicide | 60mg Yohimbe | 120mg Yohimbe | 180mg Yohimbe | SEM |
| pH | 5.946 | 5.930 | 5.972 | 5.924 | 5.945 | 0.017 |
| Moisture content (%) | 74.828 | 74.597 | 74.089 | 73.449 | 73.958 | 1.627 |
| Dry matter (%) | 25.172 | 25.403 | 25.911 | 26.551 | 26.042 | 0.553 |
| Fat content (%) | 4.186 | 4.320 | 4.523 | 4.596 | 4.443 | 0.177 |
| Ash (%) | 1.170 | 1.260 | 1.240 | 1.293 | 1.270 | 0.052 |
| Crude Fibre (%) |
0.878 a |
0.890 a |
0.821 b |
0.881 a |
0.860ab |
0.008 |
| Crude protein (%) | 18.648 | 18.653 | 19.024 | 19.468 | 19.146 | 0.323 |
| Carbohydrate (%) | 0.290 | 0.280 | 0.303 | 0.313 | 0.323 | 0.017 |
a,b Means in a row with different superscripts differ significantly (P<0.05). SEM: Standard Error of Mean.
Table 7: Effects of supplemented diets on the meat lipid profile of chicken.
| Parameters | Control | Larvicide | 60mg Yohimbe | 120mg Yohimbe | 180mg Yohimbe | SEM |
| Total Cholesterol (mg/dl) | 77.67 | 79.33 | 89.33 | 80.33 | 75.33 | 3.014 |
| Triglyceride (mg/dl) | 81.33 | 66.00 | 64.33 | 66.33 | 72.67 | 2.699 |
| HDL (mg/dl)* | 41.67 | 47.33 | 54.00 | 46.00 | 44.67 | 2.427 |
| LDL (mg/dl)* | 19.67 | 19.00 | 22.33 | 20.67 | 16.33 | 1.287 |
| VLDL (mg/dl)* | 16.00 | 13.33 | 13.00 | 13.33 | 14.67 | 0.511 |
a,b Means in a row with different superscripts differ significantly (P<0.05). *HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; VLDL: Very Low Density Lipoprotein; SEM: Standard Error of Mean.
Effect of supplemented diets on sensory scores of meat from broiler chicken
The effects of supplemental diets on the sensory scores of meat of broiler chicken is presented in Table 5. There were no significant difference (P>0.05) in the colour, saltiness, and overall flavour of chicken fed the experimental diets. However, chicken fed Basal diet + 120mg Yohimbe were significantly (P<0.05) less Juicy and Tenderness while chicken fed Basal diet + 60mg Yohimbe had significantly (P<0.05) less meaty flavour and overall acceptability.
Meat Proximate composition and pH value of chicken fed supplemented Yohimbe
Table 6 shows the effect of supplemented diets on the pH and proximate composition of broiler chicken. There were no significant difference in the pH, moisture content, dry matter, fat content, ash, crude protein, and carbohydrate of chicken feed the experimental diets. However, the crude fibre of chicken fed Basal diet + 60mg Yohimbe was significantly reduced (P<0.05).
Effects of supplemented diets on the lipid profile of chicken
Table 7 shows the lipid profile of chicken fed supplemented diets of broiler chicken. There were no significant difference in the total cholesterol, Triglyceride, High Density Lipoprotein (HDL), Low Density Lipoprotein (LDL), and Very Low Density Lipoprotein (VLDL) of chicken fed experimental diets.
DISCUSSION
The non-response in the relative weights of bled and dressed carcass, drumstick, back, breast, wings, crop, caecum with gizzard width and length to Yohimbe supplements could be due to the levels of inclusion. This report is similar to the findings of Metin and Ahmet (2016) who supplemented Yohimbe extracts in chicken drinking water. However, inclusion of Yohimbe increased the relative weights of heart, spleen, kidney, proventiculus, duodenum and ileum + jejunum of broiler chickens. The increase in the relative kidney weight could result from the increased activity of the kidney. This includes anti-diuretic activity of the kidney being enhanced by the presence of Yhombine in the blood; as it is reported by Farjan and Grevan (1989), as well as the excretory role of the kidney as it is responsible for the removal of toxic metabolites from the system. The kidney might be further laboured in the removal of metabolites synthesized from other natural compounds in Yohimbe (Mahmoud, 2000; Chen et al., 2008). Furthermore, increase in relative heart weights can be as a result of increase in mean blood pressure and blood flow which was initiated by the presence of Yohimbe in the blood; as similar response was reported by Ajayi et al. (2003) who fed Yohimbe to Sprague Dawley rats. In addition, higher relative spleen weight could be due to increased immunoactivity in the chicken. Yohimbe has been reported to contain alkaloids which are phytobiotics Morel et al. (2005). Improved immune response has been associated with increase in relative weights of spleen and other lymphoid organs such as bursa and thymus (Alkhalf et al., 2010). The pancreas is responsible for hormone production and the digestion of fats. The increased relative pancreas weights with the inclusion of Yohimbe might suggest Yohimbe stimulate secretion of digestive fluid from the pancreas.
Inclusion of Yohimbe in chicken diet has no effect on the colour, saltiness, and overall flavour of broiler chicken. The non-response of colour to the supplementation shows Yohimbe does not contain natural pigments like carotenoids and xanthophylls, neither does it influence the heme pigmentation, sugar content, nor amino acid profile of the chicken tissues (Nasir et al., 2017). Also, qualities like juiciness, meaty flavour and overall acceptability were grossly improved. The origin of meat flavour is the reaction between the products of lipid oxidation, Maillard’s reaction and degradation of vitamins during cooking (Khan et al., 2015). The improvement in the meaty flavour may be traced to the improvement in the crude protein content and the intramuscular fat of the chicken muscles with the inclusion of Yohimbe. Yohimbine has been reported by Metin and Ahmet (2016) to increase muscle protein content which may further contributes to the meaty flavour. In addition, the variation in the tenderness of the chicken meat may be as a result of the changes in the fibre content of the chicken breast. As Nasir et al. (2017) suggests the connective tissues have a major role in the determination of meat tenderness. Furthermore, changes in the juiciness might be as a result of variation in the meat fat content in the form of intramuscular fat as Calkins and Sullivan (2007) submits that the lubrication provided by marbling could increase the perception of tenderness and juiciness of meat. The overall acceptability of chicken meat was improved because there were improvements in the qualities that predict palatability which include: Juiciness, tenderness, and flavour (Osadebamwen, 2015).
The inclusion of Yohimbe did not affect the meat pH and most of the proximate parameters. However, there was increase in the fat content and Carbohydrate content in meat of chicken fed Yohimbe. The increase in the fat content is at variance with the finding of Metin and Ahmet (2016) who supplied Yohimbine to chicken via its drinking water and observed a decline in lipid content. This variation may be due to the method of Yohimbe administration.
Lastly, Yohimbe has no effect on meat lipid profile when supplemented in chicken feed. This agrees with the findings of Sax (1991) who reported Yohimbine had no effect on body fat and cholesterol.
CONCLUSION
Supplementation of chicken diet with Yohimbe has no influence on the weights and cut parts. However, the supply of chicken diet with 180mg/Kg of Yohimbe improved the sensory properties of chicken meat like juiciness, meaty flavour and overall acceptability. In addition, supplementation of chicken diet with Yohimbe has no influence on most of the proximate composition but supplementation with 60mg/Kg Yohimbe gives meat with the least fibre content.
Acknowledgements
The authors duly acknowledged the research support and financial assistance provided by the World Bank through the Centre of Excellence in Agricultural Development and Sustainable Environment (CEADESE) of the Federal University of Agriculture, Abeokuta.
Authors Contribution
O.V. Obajuluwa: Partake in conducting farm experiment as well as writing and revision of the final manuscript. K.A. Sanwo: Partake in conducting the meat quality analysis plus the revision of the final manuscript. L.T. Egbeyale: Partake in designing and conducting of the farm experiment as well as the editing of the paper. A.O. Fafiolu: Conducting the statistical analysis of the data
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






