Journal of Animal Health and Production
Research Article
Occurrence of Ectoparasites in Backyard Domestic Chickens (Gallus gallus domesticus) in the Northeast of Tunisia
Khaled Kaboudi1*, Rafika Ben Romdhane2, Ameni Ben Salem3, Moncef Bouzouaia1
1Department of Poultry Farming and Diseases, National Veterinary Medicine School of Sidi Thabet; University of Manouba, Tunisia; 2Department of Animal Production, Regional District of Agricultural development of Tunis – Tunisia; 3National Center of Zoosanitary Eve of Tunisia, Tunisia.
Abstract | The presence of ectoparasites on birds can lead to low productivity and eventually death. This study was carried out to identify and estimate the prevalence of ectoparasites of free-range poultry (Gallus gallus), in the Northeast of Tunisia. A total of 512 birds of both sexes and aged from 1.5 month to 24 months were examined and/or necropsied. Results showed that 19 ± 3.39% (98 birds), as overall prevalence, were infested by at least one parasite specie. Young animals were more infested (13 ± 2.91%) than adults (6 ± 2.05%). There was significant difference (P < 0.05) in prevalence found during rainy and cold seasons (winter: 24 ± 6.59%; spring: 23 ± 7.83%; autumn: 20 ± 7.87%). Six species of ectoparasite were identified in the present study. Cnemidocoptes mutans (8 ± 2.35%) was the most prevalent, followed by Menopon gallinae (6 ± 2.05%), Laminosioptes cysticola (3 ± 1.47%), tick larvae of Argas persicus (3 ± 1.47%), Echidnophaga gallinacea (3 ± 1.47%) and Menacanthus stramineus (2 ± 1.22%). Infestation by one species of ectoparasite (13 ± 2.91%) was more frequently than mixed infestation (6 ± 2.05%). On the basis of these results it could be suggested that, good management, biosecurity and the education of farmers could be a vital measures to keep free from ectoparasites and improve the productivity of the chicken.
Keywords | Backyard poultry, Ectoparasite, Prevalence, Infestation, Tunisia
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | February 25, 2019; Accepted | June 18, 2019; Published | September 15, 2019
*Correspondence | Khaled Kaboudi, Department of Poultry Farming and Diseases, National Veterinary Medicine School of Sidi Thabet; University of Manouba, Tunisia; Email: kaboudikhaled17@gmail.com
Citation | Kaboudi K, Romdhane RB, Salem AB, Bouzouaia M (2019). Occurrence of ectoparasites in backyard domestic chickens (gallus gallus domesticus) in the northeast of tunisia. J. Anim. Health Prod. 7(3): 92-98.
DOI | http://dx.doi.org/10.17582/journal.jahp/2019/7.3.92.98
ISSN | 2308-2801
Copyright © 2019 Kaboudi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Backyard poultry sector is considered as an important source of income for several families in rural areas in Tunisia. These flocks provide a cheap source of protein in the form of meat and eggs. This traditional production contributes to the national poultry production with average of 7% (GIPAC, 2010).
Several factors limit the productivity in free-range poultry flocks. Malnutrition, poor management, lack of biosafety conditions and poor vaccination plans. Losses have also been attributed to limit housing and specific veterinary care services. Furthermore, poor genetic potential due to lack of selection and predation are also potential threats to productivity. Parasitic infestation is considered as an important health problem in free-range poultry which handicaps production (Mungube et al., 2008).
Birds reared in the free range system are at constant risk of infestation by several types of endoparasites and ectoparasites. Several, species of ectoparasites (flies, lice, mites, and ticks) can infest poultry (Permin et al., 2002). They cause discomfort, decrease in growth and egg production, anaemia, irritation, loss of plumage and skin lesions that may be site of secondary infection. Death may occur when infestation is massive. Environments with high parasitic pressure can limit immunity protection against infectious diseases. A number of epidemiological factors including host, sex, age, breed and environment may influence the occurrence
Table 1: Prevalence rate of ectoparasites in male, female, young (≤ 18 weeks) and adult (> 18 weeks) free-range poultry
| Sex |
Age |
|||||
| Male |
Female |
χ2 (p-value) |
Young |
Adult |
χ2 (p-value) |
|
| Number of examined | 151 |
361 |
1.02 (p=0.312) | 382 | 130 | 4.389 (p=0.036)* |
| Number of positive | 33 | 65 | 65 | 33 | ||
| Prevalence (%) | 22 ± 6.60% | 18 ± 3.96% | 17 ± 3.77% | 25± 7.44% | ||
* = significant difference
Table 2: Prevalence rate of ectoparasites in various types of free-range poultry
| Broiler |
Pullet |
Layer |
Rooster |
χ2 (p-value) |
|
| Number of examined | 138 | 244 | 117 | 13 | 57.24 (p<0.001)* |
| Number of positive | 20 | 45 | 20 |
13 |
|
| Prevalence (%) | 14 ± 5.79% | 18 ± 4.82% | 17± 6.80% | 100 ± 23.07% |
* = significant difference
Table 3: Season-wise prevalence of ectoparasites in free-range poultry
|
Season |
Number of examined |
Number of positive |
Prevalence (%) |
χ2 (p-value) |
|
Autumn |
99 |
20 |
20 ± 7.87% |
9.549 (p=0.022)* |
|
Winter |
161 |
38 |
24 ± 6.59% |
|
|
Spring |
111 |
25 |
23 ± 7.83% |
|
|
Summer |
141 |
15 |
11 ± 5.16% |
|
|
Total |
512 |
98 |
19 ± 3.39% |
* = significant difference
and intensity of parasitic infestations (Nadeem et al., 2007).
Ectoparasites feed on blood, feathers, skin and scales of their host. They may transmit several infectious diseases and serve as transport or intermediate hosts for different helminthic parasites (Hopla et al., 1994). Menacanthus stramineus, known to feed on blood, can carry the equine encephalomyelitis virus. Chlamydophila psittaci, has been isolated from Menopon gallinae (Calnek et al., 1991). Many other poultry pathogens, such as Pasteurella, fowl pox, Newcastle disease virus, can be spread by ticks and mites (Nnadi and George, 2010). Dermanyssus gallinae has been widely reported to transmit human and animal pathogens (e.g., viruses and bacteria) and parasites (e.g., Hepatozoon) to persons (Valiente et al., 2009).
The present study was undertaken with the aims of determining the prevalence rates of ectoparasitic species in free-range backyard chickens in the Northeast regions of Tunisia.
Materials and methods
This study was conducted, from September 2011 to June 2015, on 512 local chickens. Animals are from regions rounding the town of Sidi Thabet, in the Northeast of Tunisia. All birds were from farms in which the traditional breeding is practiced. They were of both sexes and aged from 1.5 to 24 months. These animals were divided into two groups, namely young and adult.
The birds were received for clinical exam and/or necropsy in the Avian Clinic of the National Veterinary Medicine School of Tunisia. External parasites were collected from different parts of the body around the wing, head, vent, feather, feet, leg wattle and comb. The legs and featureless areas with crest were scrapped for parasitological identification. Parasites were identified according to their morphological characteristics using the key criteria as mentioned by Soulsby (1982).
Statistical Analysis
Comparative analysis of prevalence in chickens was performed using the chi-square test (Word Excel, Microsoft office, version 2013). A p-value less than or equal to 0.05 were used as a limit of statistical significance.
Results
Overall, 98 (19 ± 3.39%) of the examined birds were found to be infested with at least one ectoparasite specie. The infestation was predominated in male (22 ± 6.60%) (χ2= 1.02; p=0.312). The infestation was significantly higher in adult birds (25± 7.44%) than in young (17 ± 3.77%) (χ2 = 4.389; p=0.036) (Table 1).
Table 4: Prevalence of individual parasites in female, male, young (≤ 18 weeks) and adult (> 18 weeks) free-range poultry
|
Parasite species |
Overall prevalence |
No. positive (%) |
χ2 (p-value) |
No. positive (%) |
χ2 (p-value) |
||
|
Female |
Male |
Young |
Adult |
||||
|
Cnemidocoptes mutans |
8 ± 2.35% |
12 (12 ± 6.34%) |
31 (32 ± 9.23%) |
50.636 (p<0.001)* |
18 (18 ± 7.6%) |
25 (26 ± 8.57%) |
20.535 (p<0.001)* |
|
Menopon gallinae |
6 ± 2.05% |
15 (15 ± 7.07%) |
14 (14 ± 6.87%) |
3.932 (p=0.02)* |
20 (20 ± 7.92%) |
9 (9 ± 5.66%) |
0.128 (p=0.5) |
| Menacanthus stramineus |
2 ± 1.22% |
5 (5 ± 4.31%) |
7 (7 ± 5.05%) |
3.723 (p=0.05)* |
4 (4 ± 3.88%) |
8 (8 ± 5.37%) |
6.664 (p=0.001)* |
|
Echidnophaga gallinacea |
3 ± 1.47% |
4 (4 ± 3.88%) |
10 (10 ± 5.94%) |
10.424 (p=0.001)* |
11 (11 ± 6.19%) |
3 (3 ± 3.37%) |
1.096 (p=0.2) |
|
Laminosioptes cysticola |
3 ± 1.47% |
10 (10 ± 5.94%) |
7 (7 ± 5.05%) |
0.518 (p=0.3) |
9 (9 ± 5.66%) |
8 (8 ± 5.37%) |
1.65 (p=0.1) |
|
Argas persicus |
3 ± 1.47 % |
8 (8 ± 5.37%) |
9 (9 ± 5.66%) |
3.419 (p=0.05)* |
6 (6 ± 4.7%) |
11 (11 ± 6.19%) |
8.868 (p=0.001)* |
* = significant difference
Different types of poultry were infested as indicated in Table 2. However, it was remarkable that all roosters were positive (100%), followed by pullets (18 ± 4.82%), layers (17± 6.80%) and broilers (14 ± 5.79%). The relationship between infestation prevalence and type of production was highly significant (χ2=57.24; p<0.001).
The study according the season is summarized in Table 3. The results showed that parasitic infestations were diagnosed along the year, with statistically significant (χ2=9.549; p=0.022) most high incidence observed during winter (24 ± 6.59%), spring (23 ± 7.83%) and autumn (20 ± 7.87%). Influence of sex (χ2=9.495; p=0.02), age (χ2=14.713; p=0.001) and type of production (χ2=29.566; p<0.001) on seasonal repartition was statistically significant.
Regarding to the importance of infestation, highest prevalence of single infestation (67 birds; 13 ± 2.91%) was revealed compared to mixed infestation (31 birds; 6 ± 2.05%). This repartition was influenced by the age (χ2=10.149; p<0.05) of birds but not by the sex factor (χ2=0.515; p=0.47). Study of mixed infestation showed a prevalence rate of 5 ± 1.88% (28 birds) and 1 ± 0.86% (3 birds) in chickens infested by two and by three species of ectoparasites, respectively. No animals infested by up than three ectoparasites in this study.
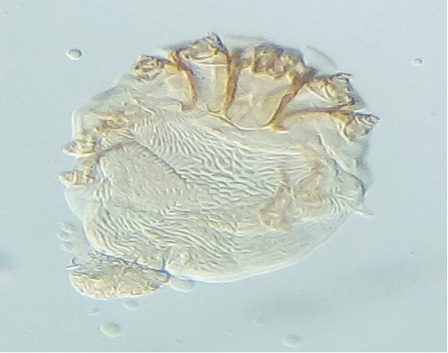
Six ectoparasite species were identified in the present study. Results of the individual prevalence of each parasite depending on sex and age were summarized in Table 4. The most prevalent species was Cnemidocoptes mutans (8 ± 2.35%) (Figure 1), followed by Menopon gallinae (6 ± 2.05%) (Figure 2), tick larvae of Argas persicus (Figure 3), Laminosioptes cysticola (Figure 4) and Echidnophaga gallinacea with the same percentage (3 ± 1.47%). Whereas, Menacanthus stramineus (2 ± 1.22%) (Figure 5) was the least prevalent ectoparasite, identified in backyard chickens in the studied region.

Figure 2: Menopon gallinae in free-range chicken
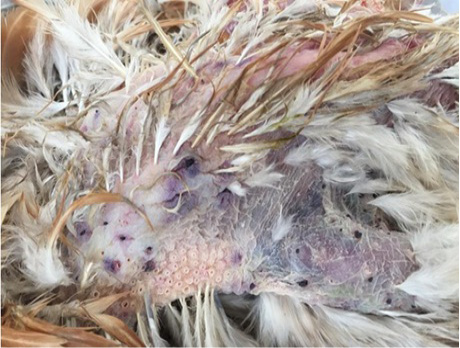
Figure 3: Severe infestation of a free-range chicken by larvae of Argas persicus. (Note hemorrhage zones corresponding to the points of attachment of tick larvae)

Figure 4: Calcified cysts of Laminosioptes cysticola (arrows) in the subcutaneous collagen in the region of breast
Discussion
Results of this study show overall prevalence assessed to 19 ± 3.39% (98 animals out of 512 examined) in the northeast region of Tunisia. High prevalence was found in other countries, such as Zimbabwe (Goromonzi District) (100%) (Permin et al., 2002), Potiskum (Nigeria) (84.50%) (Lawal et al., 2017), Benue State (Nigeria) (69.7%) (Oche et al., 2016), West of Iran (64.83%) (Rezaei et al., 2016) and Algeria (69-100%, according to the region study) (Meguini et al., 2018). Infestation by ectoparasites is favorited in free range farming system by several factors, such as possible contact of chickens with animals of other poultry species and wild birds (Adelusi et al., 2014).
Prevalence of the ectoparasites infestation was found to be statistically higher in adult (25± 7.44%) than in young. Malann et al. (2016), Oche et al. (2016), Kebede et al. (2017) and Lawal et al. (2017) have also reported that adult village chickens were more infested by ectoparasites compared with younger ones. This finding might be associated with the frequent contact of adult chickens with other species of animals and may be exposed longer to the infested environment and other source of infestation. These findings were in coherence with prevalence rate calculated in different type of production. In fact, roosters (100%) and pellets (18 ± 4.82%) were statistical significantly more frequently infested by ectoparasites than layers (17± 6.80%) and broilers (14 ± 5.79%).
Our results regarding the age of animal were in disagreement with the findings of Mulugeta et al. (2013), Firaol et al. (2014) and Rezaei et al. (2016) who reported that young chickens were more infested than adult animals.
There is no affinity of ectoparasites to the sex of animals, which is in disagreement with the findings of Tolossa et al. (2009). Indeed, theses authors demonstrated higher significance infestation of male (93.94%) compared to female (76.19%). Prevalence of ectoparasitic infestation was statistical significantly higher in raining and humid season (autumn, spring and winter), with global prevalence assessed to 22 ± 4.21%, which is in agreement with the observations of Mukaratirwa and Hove (2009) and Mohammad et al. (2016) who also reported high prevalence of ectoparasites in chickens during the rainy season. Generally, the rainy season consists of favorable climatic conditions for the proliferation of parasites. Moderated to high ambient temperature and humidity during this season are very essential for the hatching of eggs and larval developmental stages (Soulsby, 1982). These climatic characteristics explain that external parasites of poultry are common in the tropics (Nnadi and George, 2010; Firaol et al., 2014). Although, ectoparasites infestation have also been reported during the dry season (Alemu et al., 2015; Kebede et al., 2017).
Single ectoparasite infestation (13 ± 2.91%) found in this study was more frequent than mixed infestation (6 ± 2.05%). These findings are not in line with those of several authors, such as Medjouel et al. (2013), Mulugeta et al. (2013), Firaol et al. (2014) and Lawal et al. (2017), who reported higher prevalence of mixed ectoparasitic infection in village chickens. The difference may be associated with different agro-ecology, climatic factors in the various study areas, variation in sample size and sampling period, free range nature of village chickens, level of hygiene in and around the poultry houses and the level of parasite control practices.
Six species of ectoparasites were identified in the present study, which included Cnemidocoptes mutans, Menopon gallinae, Menacanthus stramineus, Echidnophaga gallinacea, Laminosioptes cysticola and tick larvae of Argas persicus. Among these species of ectoparasites encountered, Cnemidocoptes mutans (8 ± 2.35%) was the most prevalent, while Menacanthus stramineus (2 ± 1.22%) was the least. The prevalence rate of Cnemidocoptes mutans was higher than that mentioned by Lawal et al. (2017) (7%) and Odenu et al. (2016) (7.26%). Nevertheless, it was lower than prevalence rate found by Bala et al. (2011) (9.40%), Firaol et al. (2014) (34.62%) and Oche et al. (2016) (18.18%). Cnemidocoptes mutans is a scale leg mite; a small spherical sarcoptic mite usually tunnels in to the tissue under the scales causing an inflammation and market keratinization, which is responsible for the thickened scaly nature of the feet. Low prevalence revealed in our study compared to the prevalence reported by Hagos and Eshetu (2005) (19.5%), may be due to the cold temperature and/or health management system.
Among the lice species observed, Menopon gallinae and Menacanthus stramineus. The prevalence rate of Menopon gallinae from the present study was 6 ± 2.05%, which were considered higher than that found by Lawal et al. (2017) in Nigeria. However, our result was lower than 13.28%, 8.1%, 12.4%, 40.12%, 97.2% and 97.7%, reported by Kebede et al. (2017), Bala et al. (2011), Moyo et al. (2015), Sadiq et al. (2003), Medjouel et al. (2013) and Sabuni et al. (2010), respectively. Menacanthus stramineus was detected in 2 ± 1.22% of examined chickens. Its prevalence rate was lower than results reported by several authors in different countries, such as Belihu et al. (2010), Bala et al. (2011), Medjouel et al. (2013), Moyo et al. (2015) and Mirullo and Mullens (2016) in Ethiopia, Nigeria, Algeria, South Africa and California, respectively. This parasite is the most pathogenic in poultry. It causes severe anemia in the host by feeding on blood that oozes out. It also causes inflammation of skin and extensive scab formation. However, exact impact of lice species on the chicken was not determined.
The prevalence rate of Laminosioptes cysticola was 3 ± 1.47%. This ectoparasite was rarely described in published studies. Considered as a parasite of the subcutaneous and inter-muscular collagen tissue, it is generally not pathogenic in poultry. However, this parasite may represent a risk of food-borne human allergy. Infestation induce the formation of white calcified nodules observed in the necropsy exam or after slaughter in the internal face of thigh, on the breast and the neck and on the internal viscera (Da Silva Martins et al. 2010). Sometimes, severe infestation can induce clinical illness. In this way, Smith et al. (1997) described nervous signs (torticollis, circling, loss of balance, wing droop) in wild turkey massively infested with Laminosioptes cysticola. Indeed, granulomatous inflammation can be abundant in brachial plexus and sciatic nerves.
Tick larvae infestation were represented by Argas persicus in 3 ± 1.47% of cases. This prevalence was lower than that mentioned by Lawal et al. (2017) (6.2%) and Rezaei et al. (2016) (78.66%) in Nigeria ans Iran, respectively. High risk of infestation was related to the presence of other animal species (cattle, dog...) in the farm. In this condition, ticks causes blood spoliation leading anemia and death. Argas persicus can cause paralysis in infested birds and a decrease in egg production. Indirect pathogenic action of ticks is explained by their role of pathogens vector. Argas spp can infest man, especially in rural areas, where there close contact between man and animals. They cause irritation and allergic problems (Sadiq et al., 2009). In fact, theses parasites can transmit bacterial, rickettsial, viral, parasitic and spirochaetal diseases in poultry (Haider Shah et al., 2004). In the same way, Argas persicus larvae have been responsible for episodes of infectious bursal disease and spirochaetosis (Abdu, 1987).
Among the identified flea species, Echidnophaga gallinacea was detected in 2 ± 1.22% of examined birds. The prevalence of this ectoparasite in our study was not similar to those reported from west of Iran with 6% (Rezaei et al., 2016), 18% in California (Murillo and Mullens, 2016), 29.6% in Abuja (Nigeria) (Odenu et al,. 2016), 27.3% in Gombe (Nigeria) (Lawal et al., 2016) and from different regions in Ethiopia with 26.6% (Mata et al., 2018) to 51.3% (Belihu et al., 2009). Echidnophaga gallinacea attaches to the combs, wattles and around the eyelids, where it induces irritation, restlessness and anemia (Soulsby, 1982). Our study excludes many other parasites feeding only briefly on birds, such as Goniocotes gallinae, Lipeurus caponis and Dermanyssus gallinae.
Finally, it is important to mentioning that variations in the prevalence rates and diversity of species of ectoparasites from the reports of the various studies may be connected to several factors. The differences in geographical areas, host factors, husbandry and management system, poor sanitation, sample size, period of study as well as different favorable climatic conditions such as temperature and humidity which may influence the population dynamics of the ectoparasites are the most included epidemiological factors (Arends, 2003; Prelezov and Kolnarski, 2006).
Conclusion
This study showed that backyard chicken could be infested by several species of ectoparasites in the regions around Sidi Thabet in the Northeast of Tunisia. Parasitic infestation affect health status of animals and their productivity. Several risk factors can influence the prevalence and the severity of infestation, such as age, sex and season. Poor management conditions, mixed animal and avian species and negligence of antiparasitic application can preserve the infestation in the flock. That is why, the association of insecticide treatments to the hygiene are crucial to minimize damages and preserve the traditional poultry. Further studies are needed to determine the economic impact of ectoparasitic infestation in backyard flocks and to perform global strategy of control.
Conflict of Interest
There is no conflict of interest.
Authors Contribution
Khaled Kaboudi: Sampling, statistical analysis, interpretation and redaction.
Rafika Ben Romdhane: Sampling and redaction.
Ameni Ben Salem: Sampling and interpretation.
Moncef Bouzouaia: Interpretation.
References