Advances in Animal and Veterinary Sciences
Research Article
Silver Nanoparticles Embedded Photosensitive Silicon as Catheter Materials
Swati Dahiya1*, Neha Bhardwaj2, Sanjeev Bhardwaj2, Jyotsana Mehta2, Arun Sehgal, Minakshi Prasad3
1Department of Veterinary Microbiology, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar-125004, Haryana; 2Central Scientific Instrumentation Organisation (CSIO), Sector-30C, Chandigarh-160030; 3Department of Animal Biotechnology, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar-125004, Haryana, India.
Abstract | Biofilm growth on catheter surface is a challenge for various biomedical applications. Microbial colonization on catheter’s surface leads to many infections. Silicone polymer is a routine material for catheter fabrication. In the present work, silver nanoparticles (NPs) and photosensitive non-toxic dye toluidiune blue (TB) embedded silicon polymer is synthesised by swell encapsulation method and characterised by UV spectroscopy. The embedded polymer showed high bactericidal activity against gram-positive bacteria Staphylococcus aureus (S. aureus) and gram-negative Proteus mirabilis (P. mirabilis) with least leaching effects which suggests their usage as a new weapon to fight catheter related infections.
Keywords | Catheter, Photosensitizer dye, Toluidine blue, Silver nanoparticles, Bactericidal
Editor | Sandip Kumar Khurana, Principal Scientist, National Center on Equines (NRCE), Sirsa Road, Hisar, Haryana, 125004, India.
Special Issue | 1(2015) “Biotechnological and molecular approaches for diagnosis, prevention and control of infectious diseases of animals”
Received | December 04, 2014; Revised | March 21, 2015; Accepted | March 22, 2015; Published | March 31, 2015
*Correspondence | Swati Dahiya, Lala lajpat Rai University of Vetrinary and Animal Sciences, Hisar, Haryana, India; Email: [email protected]
Citation | Dahiya S, Bhardwaj N, Bhardwaj S, Mehta J, Sehgal A, Prasad M (2015). Silver nanoparticles embedded photosensitive silicon as catheter materials. Adv. Anim. Vet. Sci. 3(1s): 10-15.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.1s.10.15
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Dahiya et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Catheter is a thin, flexible, sterile tube made up of flexible polymeric materials such as silicone and polyurethane having versatile biomedical applications (Trautner and Darouiche, 2004). The surface of a catheter often gets colonized by micro-organisms that form biofilms on their surface, which results catheter-associated infections (CAIs). These infections can consequently lead to destruction of tissues, systemic dissemination of the pathogen and dysfunction of the device, thus, resulting in serious illness and death of the individual (Hall-Stoodley et al., 2004). The hospital acquired bloodstream infections have a mortality rate of 10-25% and are generally caused by usage of two common types of catheter i.e. intravascular catheter and urinary catheter (Maki and Mermel, 1998). The annual usage of central venous catheters in US alone is around 5 million, of which about 3-8% result in bloodstream infections with a mortality rate of ca. 15% (Trautner and Darouiche, 2004). Also, urinary catheters are used at large scale (more than 30 million) every year in US, with an infection rate of about 10-30%, of which <1% result in death (Veenstra et al., 1999).
Most of the CAIs are caused by biofilm forming bacteria such as Escherichia coli, Pseudomonas aeruginosa, Klebsilella pneumonia, Proteus mirabilis, Staphylococcus epidermidis, Staphylococcus aureus, Enterococcus spp., Stenotrophomonas and Candida species posing a critical medical problem worldwide (Trautner and Darouiche, 2004; Tenke et al., 2004). Among these biofilm-forming bacteria, S. aureus is the most commonly found gram-positive bacterium causing 50%–70% catheter associated biofilm infections (Agarwal et al., 2010; Walz et al., 2010). In addition, there is an increasing rise of antibiotic resistance in S. aureus which further raises concern about its prevalence in medical institutes and hospitals (Chambers and DeLeo, 2009). P. mirabilis is also a biofilm forming gram-negative bacterium with the greatest ability to attach to a catheter’s surface (Robert et al., 1990; Warren 1996). It is the third most common cause of urinary tract infections (12%) and the second most common cause of catheter associated bacteriuria (15%) in long term catheterized patients. The biofilms formed by P. mirabilis on urinary catheter surfaces account for upto 30% of all urinary tract stones (Hochreiter et al., 2003). The crystalline biofilms formed by this bacterium can even lead to blockage of catheters and bladder stone formation (Sabbuba et al., 2004). Taken together, the burden on healthcare system by such biofilm forming microbes is enormous and the prevention of CAIs caused by such microbes is a major challenge in biomedical field due to the growing antibiotic resistance in micro-organisms (Gould, 2008). A multitude of methods have been described previously for prevention of such infections by many researchers but the recent methods involve modification of catheter surfaces either by coating or by impregnation of antibacterial agents or antiseptics (Siddiq and Darouiche, 2012). Catheter materials that resist encrustation by bacterial biofilms can be developed either by incorporation of an antimicrobial agent into the polymer (Darouiche and Raad, 1997) or by developing surface modified materials which prevent the adherence of bacterial cells (Stickler et al., 2002). The use of silver in particular has shown cidal effect against many of the bacteria associated with CAIs (Furno et al., 2004; Kim et al., 2007). Due to the small size and higher surface-to-volume ratio of metallic nanoparticles (e.g. silver NPs), they have an increased contact area with the microorganism and thus acquire higher antibacterial activity in comparison to the conventional antibiotics or antiseptics (Panacek et al., 2006). Also, the NPs can target different functions of bacterial cell membranes, such as permeability and respiration. Accumulation of silver on negatively charged parts of the bacterial cell membrane leads to perforation of the membrane, leakage of the cellular compounds and finally cell death. In addition, NPs can penetrate inside bacterial cell and disturb cell functions by reacting with phosphorous containing compounds such as DNA or sulphur containing proteins (Gordon et al., 2010; Singh et al., 2008). The decreased probability of developing NP resistance in bacteria due to complex action mechanisms also makes their usage better in comparison to traditional antibiotics (Chopra, 2007). The silver NPs are being used to control bacterial growth in a variety of applications such as dental, wound dressings as well as catheters (Catauro et al., 2004; Crabtree et al., 2003; Payne et al., 2009). Many research groups have demonstrated the reduction in incidence of nosocomial urinary tract infections due to antimicrobial activity of the silver nanoparticles (NPs) and silver alloys in urinary catheters (Madeo et al., 2004; Lajcak et al., 2013; Woodyard et al, 1996).
Recently, a new approach to biofilm prevention, based on lethal photosensitization is also being explored. When a photosensitiser dye is exposed to light of appropriate wavelength, it produces a highly reactive oxygen species which destroys the micro-organisms present on catheter surface (Perni et al., 2009b; Perni et al., 2010). Several organic photosensitiser (dyes) have been reported as light activated antimicrobial agents (LAAA) by embedding them inside a polymer for their antimicrobial activity against S. aureus, S. epidermidis, E. coli and P. aeruoginosa (Perni et al., 2009a; Perni et al., 2009b; Perni et al., 2010; Perni et al., 2011).
In the present work, synergistic effect of TB dye and silver NPs has been reported for antibacterial activity of silicone polymer based catheters having hybrid properties of both silver nanoparticles and photosensitizer dyes as an effective strategy for preventing CAIs.
Materials and Methods
Materials Used
Silicone nipples (Bonne, Tender Flo) were used as the source of liquid silicone polymer/elastomer. The silver nanoparticles (Central Drug House (P) Ltd) and powdered toluidine blue (TB) (S-D Fine Chemicals Limited) were used for embedding inside polymer. A low power laser source (laser diode, wavelength 630-680 nm) was used for the excitation of TB dye. LR grade acetone (S-D Fine Chemicals Limited) and phosphate buffer saline (PBS; HiMedia Laboratories Pvt. Ltd) were used in the study. The bacterial cultures i.e. Staphylococcus aureus (MTCC No. 3160) and Proteus mirabilis (MTCC No.425) were procured from Institute of Microbial Technology, Chandigarh and maintained on nutrient agar (HiMedia Laboratories Pvt. Ltd.).
Methods
Embedding of Polymer with Dye and Nanoparticles
The silicone polymer was first cut into square coupons of size around (1x1) cm2 or (2x2) cm2 or circular discs. Then, TB dye (700 ppm) and silver NPs (100 pm) were incorporated in the silicone polymer coupons by swell-encapsulation-shrinkage method (Perni et al., 2009a).
Characterisation of Embedded Polymer
The silicone polymers embedded with TB dye and silver NPs were characterised by analysing their absorbance spectrum using UV-VIS spectrophotometer (Specord-205, Analytic Jena, Germany).
Antibacterial Activity of Polymers
The antibacterial effects of silicone polymer embedded with TB dye and silver NPs were studied on S. aureus and P. mirabilis by viable cell counting method. For this, duplicate samples of polymer coupons impregnated with TB dye and silver NPs were taken and 250 µl aliquots of the bacterial suspension of S. aureus and P. mirabilis (106cfu/ml) were carefully dropped over the surface of each polymer under sterile conditions. Samples were then irradiated with laser light after different time intervals 2 minutes (L2), 5 minutes (L5), 7 minutes (L7) and 10 minutes (L10) along with the controls. 150µl of each bacterial suspension was removed from polymer sample and serially diluted (1:10) in sterile PBS each time (i.e. from control as well as embedded polymer sample) and then plated onto NA plates. All the plates were then incubated at 37oC for 24 hours in a BOD incubator and survivors were enumerated by viable counting.
Analysis of Leaching Behaviour
The stability of the impregnated dye in the polymer was tested by analysing the leaching behaviour of the embedded polymer (Parekh and Chanda, 2007). Briefly, square samples of embedded polymer were placed in PBS at 37oC and the UV spectra of PBS solution was recorded at a time interval of 24 h for 3 days to determine the possible release of TB from the embedded samples in PBS solution. The concentration of TB in PBS was then determined on the basis of its absorbance at 660 nm.
Results and Discussion
Synthesis and Characterisation of NP and TB Dye Embedded Polymer
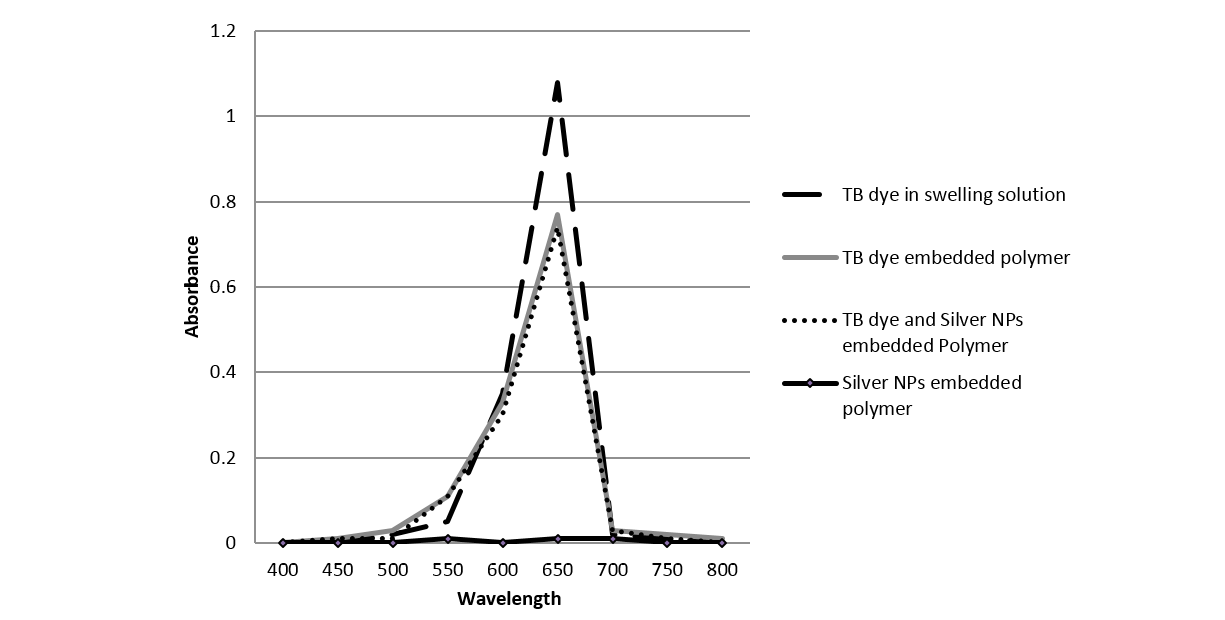
The TB (700ppm) and silver NPs (100ppm) embedded polymer was made by a swell-encapsulation-shrinkage method, which exploits the fact that silicone polymers swell when they are immersed in an organic solvent (Lee et al., 2003). This resulted in production of blue coloured polymer which shrunk to its original size and had physically encapsulated TB. The embedded polymer was characterised by UV-analysis at 660nm as the TB solution shows the main absorption peak at 660nm (Figure 1) (Perni et al., 2009a). It can be said from Fig.1 that the polymer embedded with TB only and polymer embedded with TB-silver NPs have a similar profile, confirming the presence of TB in the embedded polymer. Also, it can be observed that least absorption occurred in the polymer embedded with silver NPs alone; this is because the nanoparticles used were below the surface plasmon resonance size limit for nanoparticle colouration (Gil-Tomas et al., 2007).
Evaluation of Anti-microbial Activity of Silver NPs and TB Dye Embedded Polymer
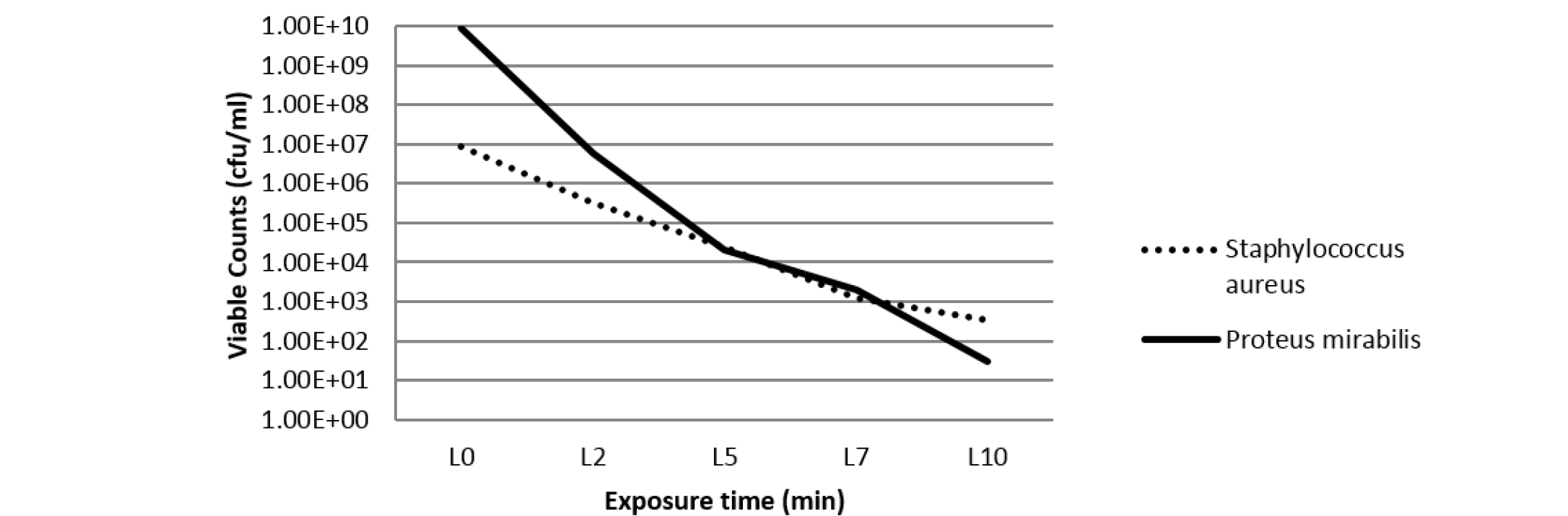
In order to evaluate the antibacterial activity of silver nanoparticles against S. aureus and P. mirabilis, agar well diffusion method was used (Parekh and Chanda, 2007). The size of zones of inhibition was measured at different concentrations of Silver NPs and 1000 ppm concentration was found most effective against S. aureus and P. mirabilis (Table 1). On decreasing the concentration of silver NPs to 100 ppm, the antibacterial activity was found to be as effective as 1000 ppm concentration of NPs against the studied bacteria. Thus, NP concentration of 100 ppm was used in the further studies. Further, the combined antibacterial activity of polymer samples embedded with toluidine blue (700 ppm) and silver nanoparticles (100 ppm) was assessed by viable cell counting method after irradiation with laser light (Perni et al., 2009a; Perni et al., 2009b; Perni et al., 2010; Perni et al., 2011). After irradiation with laser light for different time intervals, viable bacteria were counted and expressed in terms of cfu/ml (as shown in Table 2). Figure 2 shows that the concentration of viable bacteria gradually decreases with the increase in exposure time of laser light. Thus, it can be said that the antibacterial activity of embedded polymer enhances proportionally with the laser irradiation time.
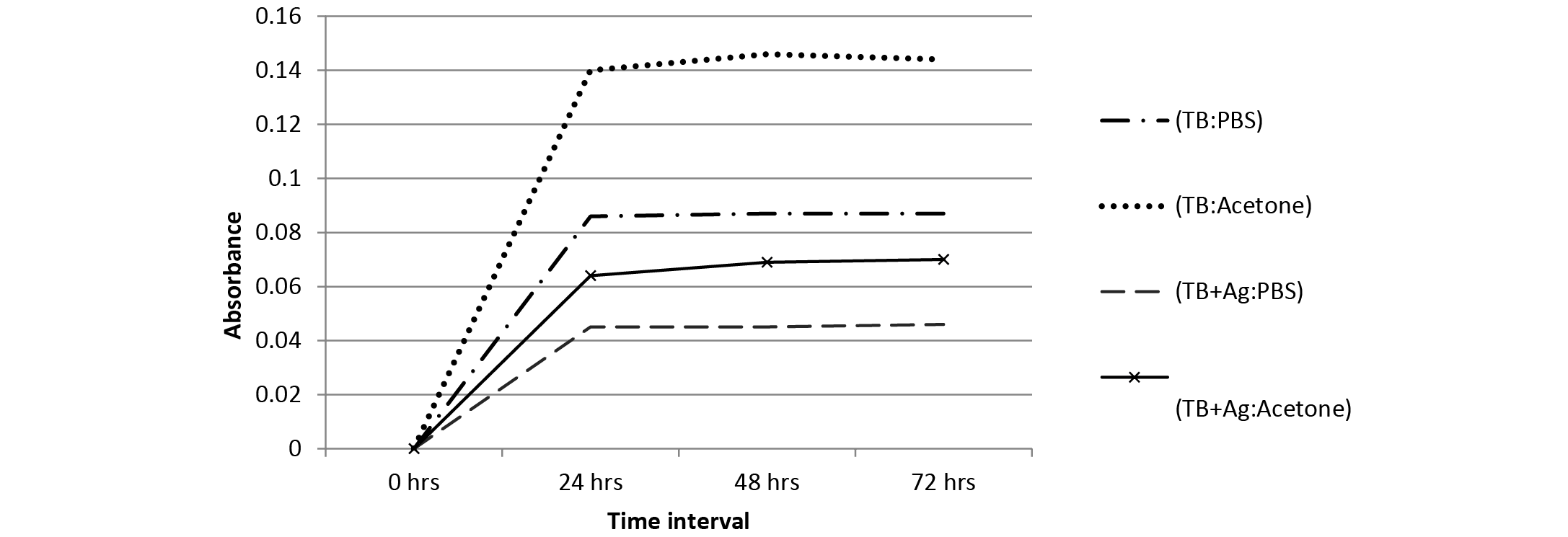
Figure 3:Absorbance spectrum of TB leaching from polymer samples in PBS and acetone with TB at 700 ppm concentration
Table 1: Antibacterial activity of silver nanoparticles at different concentrations of bacteria.
|
Concentration of silver nanoparticles |
Size of zone of inhibition (Diameter, cm) |
|
|
Staphylococcus aureus |
Proteus mirabilis |
|
|
9.2x10-3 mol L-1 (1000ppm) |
2 |
1 |
|
9.2x10-4 mol L-1 (100 ppm) |
1.8 |
0.3 |
|
9.2x10-5 mol L-1 (10 ppm) |
0 |
0 |
|
9.2x10-6 mol L-1 (1 ppm) |
0 |
0 |
Table 2: Concentration of bacterial suspensions (cfu/ml) in overnight cultures (1:1000) in PBS (std. count) and after laser irradiation for different time intervals
|
Bacteria |
cfu/ml |
||||
|
Standard count |
L2 |
L5 |
L7 |
L10 |
|
|
Staphylococcus aureus |
9 ×106 |
33×104 |
24 ×103 |
12 ×102 |
35 ×101 |
|
Proteus mirabilis |
88 ×108 |
6 ×106 |
2 ×104 |
7 ×103 |
3 ×101 |
Leaching Behaviour of Embedded Components
The leaching behaviour of TB dye (700 ppm) from embedded polymer samples was determined in both PBS and acetone using UV-VIS measurements (660 nm) at different time intervals (Figure 3). It can be observed that the leaching was less in polymer containing both TB and NPs in comparison to polymer containing TB only. Also, it can be said from Figure 3 that the leaching of TB was less in PBS as compared to acetone solution; hence the embedded polymer can be efficiently used in aqueous systems.
Conclusion
CAIs have been characterized as one of the major health problems worldwide which are difficult to treat because of the growing tolerance to antibiotics of the bacteria involved in infection. Silver nanoparticles have shown promising results as antibacterial agents owing to their free radical generation effect (Payne and Ambrosio, 2009). Also, photodynamic killing of bacteria using photosensitive dyes has been proved to be an alternative to the antibacterial drugs (Perni et al., 2010). The bactericidal activity of such dyes can be contributed to the activated oxygen species formed in response to light of certain wavelength. Thus, the present work was carried out to study the synergistic antibacterial effects of silver NPs (in low concentrations) with photosensitive dyes by embedding them in a silicone polymer. The modified silicone polymer showed a very high antibacterial activity against the studied bacteria S. aureus and P. mirabilis. Also, the polymer embedded with TB dye and silver NPs had less leaching effects as compared to the polymer containing TB dye only. Silver coated catheters have been introduced into practice and their usage in a variety of clinical settings indicates their cost-effectiveness in comparison to the costs incurred by catheter related infections (Saint et al., 1998; Karchmer et al., 2000).
Bacterial UTI is the most common infectious disease of dogs, affecting 14% of all dogs during their lifetime. The incidence of UTI is much higher in older cats as compared to young ones. The risk of urinary tract infections (UTI) among dogs hospitalized in an intensive care unit was observed as 10.3% and bacteria isolates were found common urinary tract pathogens. The UTI can further be lowered by applying properly sterilized catheter but maintenance of sterility becosmes difficult in field condition. The modified silicone polymer showed a very high antibacterial activity and can be potentially used to prevent formation of biofilm on catheter surfaces leading to urinary tract infections as well as other catheter associated infections. The use of this polymer can be potentially exploited for human and animal applications especially in pet animals such as dogs and cats (Smarick et al., 2004).
Therefore, it can be said that such polymers, impregnated with photosensitizer dyes and silver nanoparticles when periodically irradiated with short pulses of laser irradiation through an optical fibre placed in the lumen can provide a new weapon in the fight to reduce catheter related infections by inhibiting biofilm formation and surface colonization. However, further in vivo safety studies may be envisaged for their usage in practical applications.
Acknowledgements
We acknowledge Kurukshetra University, Kurukshetra for providing all the infrastructure, facilities and support during the course of research work.
References