Advances in Animal and Veterinary Sciences
Research Article
Impaired Learning and Memory after a Week Long Exposure of Acetamiprid in Adult Rats
Samiran Mondal1, Tathagata Sengupta3, Saktipada Pradhan1, Rabindra Nath Hansda1, Partha Sarathi Mandal1, Ruchi Tiwari2, Sunit Kumar Mukhopadhayay1
1Department of Veterinary Pathology, West Bengal University of Animal and Fishery Sciences, 37 KB Sarani, Kolkata-70037; 2Department of Veterinary Microbiology, College of Veterinary Sciences, Uttar Pradesh Pandit Deen Dayal Upadhayay Pashu Chikitsa Vigyan Vishwa Vidyalaya Evum Go-Anusandhan Sansthan (DUVASU), Mathura (U. P.)–281001; 3TCG Lifesciences, Saltlake, Kolkata-700091.
Abstract | Memories are thought to be encoded by modification of synaptic strength i.e. Long term potentiation (LTP), a widely considered one of the major cellular mechanisms behind the process of learning and memory. To evaluate the synaptic strength after being exposed to acetamiprid, a neonicotinoid insecticide, in animal and human, this study was taken up. In this regard, the study was conducted on 24 healthy male Sprague Dawley rats divided into two groups each having 12 animals. Group I was control and Group II served as acetamiprid treated test group. Test group animals, treated with one week exposure of acetamiprid, showed impaired learning and memory in both behavioural and functional assay tested in this experiment. Study results concluded that regular exposure to insecticide like acetamiprid impaired learning capacity and memory in rats by disrupting synaptic strength at neuronal junction of brain. Although this insecticide had shown impairment in learning and memory processes yet it remains to be learned that how this group of chemicals act. Further research need to be performed in the future to support the evidence and literature against effect of insecticide exposure in rats and in other species as well.
Keywords | Long term potentiation, Learning, memory, Acetamiprid, Electrophysiology, Hippocampus, Rat
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 18, 2014; Revised | October 27, 2014; Accepted | October 28, 2014; Published | October 30, 2014
*Correspondence | Samiran Mondal, West Bengal University of Animal and Fishery Sciences, Kolkata; Email: [email protected]
Citation | Mondal S, Sengupta T, Pradhan S, Hansda RN, Mandal PS, Tiwari R, Mukhopadhayay SK (2014). Impaired learning and memory after a week long exposure of acetamiprid in adult rats. Adv. Anim. Vet. Sci. 2 (10): 543-548.
DOI | http://dx.doi.org/10.14737/journal.aavs/2014/2.10.543.548
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2014 Mondal et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The neonicotinoids, acetamiprid and imidacloprid compounds belong to a new class of insecticide those are used worldwide to protect crops from the pest, insects and domestic animal from fleas (Mondal et al., 2012). The frequent and continuous use of acetamiprid has resulted in their widespread distribution in environment. Human and animals are indirectly exposed to different levels with long lasting behavioural changes. Earlier studies have reported memory deficit (Maren et al., 2013) in the exposed animals.
Fear is an emotion induced by a threat perceived by living entities, which causes a change in brain and that is the act of acquiring new, or modifying and reinforcing or retrieved, existing knowledge, behaviour which may involve synthesizing different types of information i. e. learning & memory. It is also essential for survival and a way of response to circuitry stimuli. The understanding of brain circuits involved in fear has been achieved by studying the circuits of learning condition for example, an environmental context (a conditioning chamber) may be arranged to deliver a stimuli in form of a foot shock, which then leads to conditioned responses to the context, such as freezing behaviour in rats (Wei et al., 2010). The hippocampus, by providing spatial and temporal information, participates in the formation of conditioned fear (Nashat et al., 2011).
In neuroscience, long-term potentiation (LTP) is a long-lasting enhancement in signal transmission between two neurons that results from stimulating them synchronously. Also it is one of several phenomena underlying synaptic plasticity, the ability of chemical synapses to change their strength. As memories are thought to be encoded by modification of synaptic strength, LTP is widely considered one of the major cellular mechanisms behind the learning and memory (Cooke and Bliss, 2006). Neuronal plasticity is the underlying property of the nervous system that enables it to adapt to various stimuli during development and adulthood. It is the basis for long-term electrophysiological changes in synaptic transmission, learning processes as well as regeneration. Long-term potentiation is a persistent synaptic enhancement induced by high-frequency stimulation of afferents and has been suggested to be an important component of the cellular basis of certain forms of learning and memory. It can be measured in different brain regions, including the hippocampus and the neocortex and it was suggested that it might be a model of processes of functional plasticity (Kato et al., 1991). LTP has been introduced in neurotoxicological studies against different environmental pollutants (Gilbert et al., 1996) and has proven to be a useful tool for linking behavioural and neurochemical outcomes.
Information is scarce in the available resourses. In this experiment a week long exposure has been made to rats with an objective to see the effects of acetamiprid on long term potentiation as well as on learning and memory behaviour. Also to explore about which of the brain structures could be affected by acetamiprid exposure and thus contribute to these long-lasting behavioral changes.
MATERIALS AND METHODS
The present study was conducted on 24 healthy male Sprague Dawley rats in two groups each group comprised of twelve (12) animals. Group I was control group which received water, while Group II served as acetamiprid treated group. The rats were procured and housed in cages in Department of Veterinary Pathology, West Bengal University of Animal and Fishery Sciences, Kolkata, India). Three animals per cage were accommodated in polycarbonate cages during the experimental period. Husk was used as bedding material. Husk was strained and sterilized by autoclaving in transparent polythene bag using sterol strip as an indicator of successful sterilization. Changing frequency of bedding material was twice a week. Animals were allowed to acclimatize for a period of 7 days prior to experiment and provided standard feed (Nutri Lab, rodent feed, Vetcare Pvt. Ltd, Bangalore) and allowed water both ad libitum (RO & UV treated).
Experimental protocol was approved by institutional animal ethical committee before starting the experiment.
Chemical and Formulation
Acetamiprid (CAS No-135410-20-7) was procured from Sigma Aldrich, USA. Acetamiprid was formulated using distilled water as a vehicle. Acetamiprid solution was administered directly in stomach by oral gavages at dose rate of 100 mg/kg (Mondal et al., 2012) with dose volume of 10 ml/kg for 7 days daily. Body weight of rats was recorded before administration of acetamiprid solution.
Contextual Fear Conditioning
This test assesses the fear condition thus providing the indication of strength of learning and memory acquired during training.
Apparatus
CoulBourn habituation instrument, Habitest isolation cubicle, USA, fitted with an electrical grid on the floor of the conditioning chamber consisted of Plexiglas box (25.0X 25.0-cm grid of parallel 0.1-cm caliber stainless steel bars 18 in number spaced 1.5 cm apart). Floor grid was removable. The electrical input can be controlled by the software graphic state. Camera is placed upside to the chamber and animal could be observed from outside over the monitor screen.
Habituation
Animals were habituated or trained for two sessions in each day on day 4 to day 6 to the context of the instrument. Each session period was for 300 seconds. In the conditioning session (training), rats were placed in the chamber for 5 min for habituation (Middei et al., 2012). During training regimen, after taking out each animal, alcohol swabbing was done to clean and remove all the dirt and smell of housed animal, so that animals in queue would not be able to assess the cue kept or left by the previous animal.
Test
After habituation in first session, on day 6, in second session an electrical shock of 2mA was given for 2 s. Animals placed to shock with the interval of 180 seconds. Before the application of shock base line freezing time (in seconds) was recorded on day 6. On day 7, total freezing duration in seconds was measured to the specified duration as it was in training session i.e. 300 seconds. Context conditioning was assessed 24 h after the training of placing rat for 5 min in the conditioning chamber (Alvares et al., 2010). Rat behaviour of fear memory was manually assessed by scoring the total amount of freezing behaviour (defined as complete lack of movement, except for respiration) during the 5-min test. Values are reported as percent of total observation time spent in freezed condition. At the end of the procedure, rat were shifted back to their respective cages.
Electrophysiology
This protocol tests the synaptic plasticity ex-vivo in the hippocampus.
Buffer: Artificial CSF (aCSF; in mM): 118 NaCl, 2.5 KCl, 25 NaHCO3, 10 glucose, 1.2 NaH2PO4, 1.3 MgCl2 and 2.5 CaCl2, under constant saturation with carbogen.
Hippocampal Slice Preparation and Electrophysiological Recording
Animals were subjected to deep anaesthesia using isoflurane (Baxtar, UK) and sacrificed by decapitation using a guillotine. Following sacrifice the brain was quickly removed and placed in chilled (4ºC) and carbogenated aCSF for 1 min. The two hippocampi were carefully dissected out from both the hemispheres and 400 µM thick transverse slices were prepared in the horizontal plane using McIlwain tissue chopper (Mickle Laboratory Engineering Co Ltd., Surrey, UK). The slices were collected in a holding chamber in aCSF under constant saturation with carbogen, where they were allowed to recover for 1-1.5 hr at 30ºC. Following recovery the slices were placed in the recording chamber, where they were continuously perfused with carbogenated aCSF (flow rate 2 ml/min) at room temperature (22-25 ºC).
Test
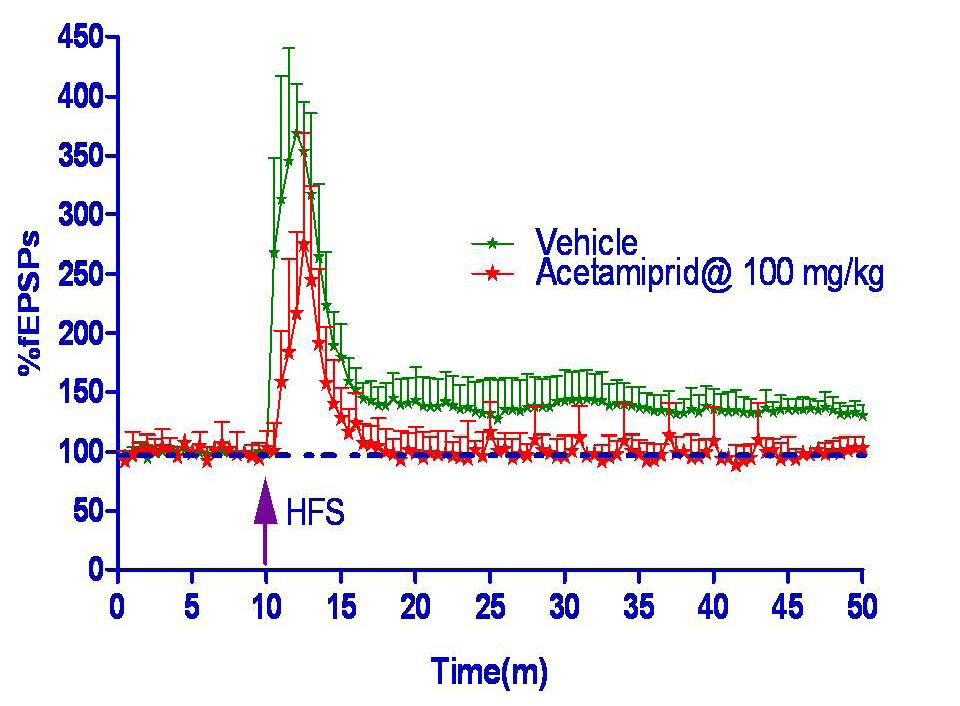
Synaptic responses were evoked by stimulating the Schaffer collateral pathway (emanating from the CA3 region) by using bipolar platinum-iridium electrode (FHC, Main, USA). The extracellular field excitatory post synaptic potentials (fEPSPs) were recorded from the stratum radiatum of the CA1 region using glass micropipette filled with aCSF. The fEPSP slope value was recorded using LTP software (WinLTP, Anderson and Collingridge, 2001). Test pulse (single current pulse of 100 µs duration at 0.033 Hz) was delivered to evoke a fEPSP. Once a response was evoked, the stimulus intensity gradually was increased to generate an Input/output (I/O) curve until a population spike was generated. The stimulus intensity was then decreased until it evoked approximately 50% of the maximal response and this intensity was used for generating a stable baseline for the whole experiment. A stable baseline was obtained for 10-15 min; LTP was induced by delivering High Frequency Stimuli (HFS; a train of 100 pulses at 100 Hz, repeated 3 times, 10 sec apart) at baseline stimulation intensity. Following HFS recording was continued using test pulse for 40 min to determine the extent of LTP generated. The percentage LTP referred to the percentage increased in the fEPSP slope values during the last 5 min of the recording period compared to the 5 min period just preceding HFS.
The evoked responses are amplified using a high-impedance differential AC amplifier (A-M Systems, Washington, USA) and digitized at 10 kHz A/D rate (National Instruments, USA).
Statistical Analysis
Statistical analysis was done by using graph pad prism 5 software. Mean differences were compared by paired t test to see the significance if any.
RESULTS AND DISCUSSION
Contextual Fear Conditioning
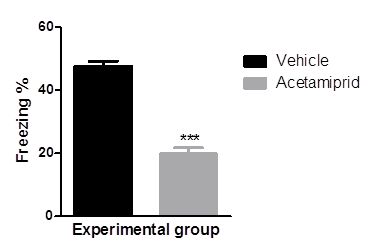
Fear to the context i.e. conditional stimuli, was measured as freezing behaviour of the individual animal. Data was represented in graphical form figure 1. Percent freezing was more in animal of vehicle group than acetamiprid treated animal. Animal in the vehicle group showed 56.4% freezing whereas acetamiprid treated animal showed only 26% freezing.
Mean values of percent freezing of experimental groups were differed significantly (p≤0.001) where Acetamiprid was administered @100 mg/kg)
Electrophysiology
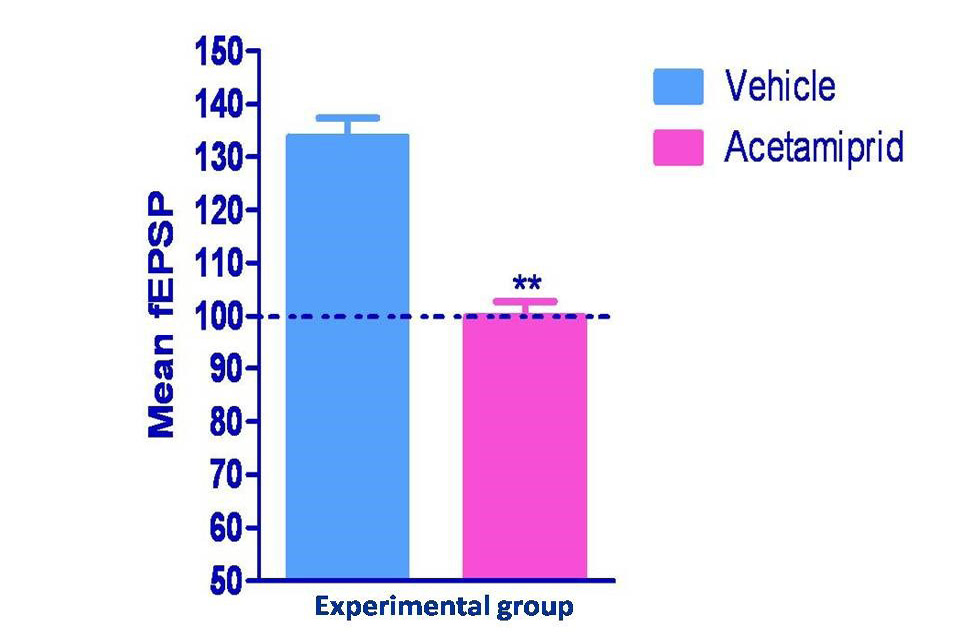
Long term potentiation measured in form of field excitatory post synaptic potential spike (fEPSPs) was shown in figure 2 and 3. Hippocampus slice from the vehicle group showed 33% LTP whereas acetamiprid group completely blocked the LTP formation.
This study showed that acetamiprid impaired learning and memory in both behavioural and functional assay designed to test in this experiment. During learning, memories are formed in a specific population of neuronal circuits that consolidated for persistence. These memory processes are supported by discrete subcellular events such as reversible modifications in the efficacy of synaptic transmission or persistent structural modifications in the size and number of synaptic connections (Aziz et al., 2014). Acetamiprid acts agonistically to neural nicotinic acetylcholine receptor (nAChR). Availability of acetamiprid in the hippocampus opens ionotropic receptors and subsequently positive ion flow into the cell causing excitotoxicity. But repetitive opening of nAChR causing desensitization to the receptor and the ion channel and thus not flowing Ca2+ across the membrane. Desensitization is a common property of nAChRs (Xiao et al., 2011). Moreover secondary inhibitory neurotransmitters like Y-amino butyric Acid, glycine released at synapse from the synoptosome causing competitive outflow with glutamate. Detrimental effect on GABAergic interneurons leads to learning and memory deficits in mice (Knoferle et al., 2014). NMDA receptor is mostly glutamate mediated, which though opened with high frequency stimulation but can’t remain open for long time. Studies performed using rat brain slices have demonstrated the acute inhibitory effect of copper on Long Term Potentation (LTP) (Leiva et al., 2009), which can be related to the effect of copper on NMDA receptor pharmacology acting as a non-competitive antagonist (Vlachova et al., 1996). The abnormal expression of NMDA receptor 2 (NMDAR 2) subunits and mGluR1 are likely to be associated with the impairment of learning and memory (Wang et al., 2013). Moreover, copper can inhibit LTP in the CA3 region of mouse hippocampus by a NMDA receptor-independent mechanism (Salazar-Weber and Smith, 2011). From this experiment it was observed that in learning and memory, nicotinic acetylcholine receptors (nAChRs) played a role (Xiao et al., 2011) and nAChR agonist like Acetamiprid impaired memory formation. Yang et al. (2012) observed thioredoxin deficit likely plays an important role in the impaired spatial learning and memory in the rats exposed to chronic intermittent hypoxia and may work through the apoptosis of neurons in the hippocampus. Research with rats indicates that spatial memory may be adversely affected by damage to the hippocampus in a way that closely resembles schizophrenia (Lewis and Levitt, 2002).
Normalized values of field potential in experimental groups were differed significantly (p≤0.01) where Acetamiprid was administered @100 mg/kg)
CONCLUSION
This study was undertaken on impaired learning and memory after a weeklong exposure of acetamiprid to adult rats. Sprague Dawley rats were used in this experiment as model animals. Regular exposure to insecticide as like acetamiprid was thought to impair synaptic strength at neuronal junction. In behavioral study acetamiprid blocked 30% memory formation and in functional assay acetamiprid completely blocked long term potentiation. Results from both assays in this experiment showed learning capacity and retention of learning in brain got hampered after exposure to acetamiprid.
REFERENCES