Advances in Animal and Veterinary Sciences
Research Article
Acaricidal Activity of Some Medicinal Plant Extracts against Different Developmental Stages of the Camel Tick Hyalomma dromedarii
Hoda S.M. Abdel-Ghany1, Sobhy Abdel-Shafy1, Mai M. Abuowarda2, Rabab M. El-Khateeb1, Essam M. Hoballah3, Magdy M. Fahmy2*
1Department of Parasitology and Animal Diseases, Veterinary Research Division, National Research Centre, Dokki, Giza, Egypt; 2Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; 3Department of Agriculture Microbiology, Agricultural and Biological Research Division, National Research Centre, Dokki, Giza, Egypt.
Abstract | The pesticidal effect of petroleum ether and ethyl alcohol extracts of Melia azedarach and Artemisia herba-alba against active stages (larvae, unfed adults and engorged females) of the camel tick Hyalomma dromedarii was evaluated by larval and adult immersion tests. Extracts were subjected to gas chromatography-mass spectrometry to identify the major active constituents. Histological sections of semifed females were evaluated to monitor the changes occurring in the cuticles and guts. The LC50 values indicated that the petroleum ether extract of A. herba-alba was more toxic against larvae (1.83%), followed by unfed adults (2.37%) and then engorged females (3.1%). However, the petroleum ether extract of M. azedarach exhibited its activity in the reverse order, i.e., it was more toxic against engorged females (3.96%), followed by unfed adults (5.47%), and then larvae 10.99%. The LC50 values of the ethyl alcohol extracts of A. herba-alba and M. azedarach on larvae, unfed adults, and engorged females were 19.60% and 22.75%, 79.81% and 19.44%, and 29.63% and 11.1%, respectively. The egg productive index (EPI), egg number, and hatchability percentage were low in the treated females. Abnormalities were observed in the cuticles and guts after treatment with plant extracts. In conclusion, the two plants M. azedarach and A. herba-alba exhibited good acaricidal activities against the active stages of H. dromedarii and might be used in the control of the camel ticks.
Keywords | Hyalomma dromedarii, Melia azedarach, Artemisia herba-alba, Medicinal plants, Acaricidal activity.
Received | February 02, 2021; Accepted | February 11, 2021; Published | March 30, 2021
*Correspondence | Magdy M Fahmy, Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; Email: [email protected]
Citation | Abdel-Ghany HSM, Abdel-Shafy S, Abuowarda MM, El-Khateeb RM, Hoballah EM, Fahmy MM (2021). Acaricidal activity of some medicinal plant extracts against different developmental stages of the camel tick hyalomma dromedarii. Adv. Anim. Vet. Sci. 9(5): 722-733.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.722.733
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Fahmy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Hyalomma dromedarii (Acari: Ixodidae) is considered the most common tick species infesting camels in Africa, Asia, and the Middle East. The adverse economic impact of H. dromedarii ticks comes from irritation, annoyance, anemia, secondary skin infection, tick fever, and is considered as a vector for various devastating diseases (Abdel-Shafy et al., 2012; Abdullah et al., 2016; Yadav et al., 2017).
Eco-friendly control of ticks is currently a crucial challenge (Benelli et al., 2016; Khater et al., 2016) where, the control of tick infestations is primarily based on the use of synthetic chemical acaricides. Due to widespread chemical applications, ticks have acquired resistance to the majority of acaricides; moreover, acaricides leave residues in foods and cause environmental pollution (Ghosh et al., 2013). Therefore, there is a need to discover eco-friendly alternatives for these chemicals. Use of herbal medications has become an attractive approach because of their biodegradability, target efficiency, and cost-effectiveness, and it has gained significant importance in tropical and subtropical regions, especially Asia and Africa (Fang et al., 2016; Niroumand et al., 2016; Qureshi et al., 2017; Showler, 2017). Plant-derived materials have been evaluated for their activity against ticks to identify compounds that can be used as substitutes for synthetic acaricides. These compounds are eco-friendly, cause the development of gradual resistance and possess various active ingredients with several mechanisms of action (Chagas et al., 2003).
Melia azedarach L. (Meliaceae) is one of the most important medicinal plants widely distributed in tropical and subtropical countries, including many African and Arab countries (Rubae, 2009). It has anti-parasitic activity against Rhipicephalus (Boophilus) microplus (Borges et al., 2003) and Dermanyssus gallinae (Sariosseiri et al., 2018). Artemisia herba-alba (known as Shieh in Egypt) is a well-known medicinal plant that is used for treating various diseases in the Middle East; as an anti-diabetic (Kamal et al., 2007; Ashraf et al., 2010) and as analgesic, antibacterial, and hemostatic agents (Mohamed et al., 2010; Tilaoui et al., 2011). It possesses acaricidal activity against Ixodes ricinus (El-Seedi et al., 2017) and H. dromedarii larvae (Abdel-Shafy et al., 2007). In a previous study the petroleum ether and ethyl alcohol extracts of these two plants exhibited good acaricidal effects against stages such as embryonated eggs and engorged nymphs of the camel tick H. dromedarii (Abdel-Ghany et al., 2019).
Encouraged by the strong effect of M. azedarach and A. herba-alba extracts on the inactive stages of H. dromedarii in our previous research, we conducted the present study to evaluate the lethal effects of petroleum ether and ethyl alcohol extracts of M. azedarach and A. herba-alba against the active stages of H. dromedarii such as larvae, unfed adults (males and females), and engorged females.
Materials and methods
Preparation of plant extracts
Dried ripened fruits of M. azedarach and aerial parts of A. herba-alba were kindly donated from the Genetics and Cytology Department, Biotechnology Division, National Research Centre. The petroleum ether (grade 40°C–60°C) and ethyl alcohol extracts were prepared according to (Borges et al., 2003; Abdel-Ghany et al., 2019) and were stored in dark glass vials at 4°C until use.
Gas chromatography-mass spectrometry (GC-MS) of petroleum ether extracts
Gas chromatography (GC) (Agilent Technologies 7890A, USA) interfaced with a mass-particular for survey just recognition (Agilent 7000 Triple Quad, USA) was used according to Abdel-Shafy et al. (2020).
Tick colony
The purpose of establishing a tick colony in the laboratory was to obtain large numbers from active stages of the camel tick H. dromedarii. Hyalomma dromedarii ticks were obtained from camels (Camelus dromedarius) in Birqash village (30°09′58.4″N, 31°02′13.2″E), Giza, Egypt during summer 2018. All ticks were identified according to the key described by Walker et al. (2003). Engorged females were incubated at 25°C ± 1°C and 75%–80% Relative Humidity (RH) separately. Eggs were collected daily and maintained in separate plastic cups to obtain larvae with the same age (Abdel-Ghany et al., 2019). The cups were tightly closed and maintained under the same condition until hatching. Some larvae were used for larval immersion test (14- to 21-day-old). Other larvae were fed on rabbits using a capsule technique (Abdel-Shafy, 2008) to obtain engorged nymphs. These nymphs were maintained under the same conditions to obtain unfed adults that were used for the unfed adult immersion test. The unfed adults were fed on rabbits to obtain engorged females that were used in bioassays.
Larval immersion test (LIT)
Initially, pilot tests were conducted for larval stage to select the suitable concentrations to be used in each extract. The test was done after the method described by Klafke et al. (2012). Approximately 300 larvae were divided into three replicates (100 per replicate) for each concentration or control. Then, the larvae were immersed in 1 ml of a tested concentration for 1 min. The concentrations of petroleum ether extract were 19.1%, 9.5%, 4.7%, and 2.4% for M. azedarach and 8%, 4%, 2%, and 1.0% for A. herba-alba. The concentrations of ethyl alcohol extract were 28.6%, 14.3%, 7.14%, and 3.5% for M. azedarach and 38.6%, 19.3%, 9.6%, and 4.8% for A. herba-alba. These concentrations were diluted in the same solvent that used in the extraction. As control, larvae were immersed in a target solvent for 1 min. As reference acaricide, larvae were immersed for 1 min in 1 ml/L of the reference acaricide Butox®5.0 (deltamethrin). Larval mortality was recorded after 24 h. Dead larvae were counted to calculate the mortality percentage. The larvae that were unable to move after stimulation by breathing or with ataxia were considered as dead. The lethal concentration (LC) values were estimate based on these mortality percentages.
Adult immersion test (AIT)
Unfed adults: A total of 30 unfed adults (10- to 15-day-old ticks) were used for each concentration or controls and divided into three replicates (10/replicate, 5 males and 5 females). These adults were immersed for 1 min in 5 ml of each concentration. The concentrations of petroleum ether extract were 38.2%, 19.1%, 9.5%, and 4.7% for M. azedarach and 16%, 8%, 4%, and 2% for A. herba-alba. The same concentrations of ethyl alcohol extracts used in AIT test were used in this test. The immersed unfed adults in the used solvent for 1 min were considered as control. As reference acaricide, unfed adults were immersed for 1 min in 1 ml/L of the reference acaricide Butox®5.0 (deltamethrin).Then, the ticks were dried on a filter paper, placed in a labeled tube, and incubated at 25°C ± 1°C and 75%–80% RH. The tubes were checked daily till 72 h to record mortality. The ticks were considered to be dead when they were unable to walk and get dark cuticles.
Engorged females: Adult immersion test carried out as mentioned by Drummond et al. (1973) with minor modification. Three replicates of five engorged females with homogeneous weight were used for each concentration. The initial weight of each female was recorded before treatment (required for calculation the egg productive index), and it was then immersed in 10 ml of a tested concentration for 1 min. The same concentrations used in AIT test were used in this experiment excepting the concentrations of petroleum ether extract of M. azedarach were 9.5%, 4.7%, 2.3%, and 1.1%. The immersed engorged females in solvents for 1 min were considered as control. As reference acaricide, engorged females were immersed for 1 min in 1 ml/L of Butox®5.0 (deltamethrin).
Subsequently, these females were dried using Whatman filter paper and placed in separate labeled cups. The cups were tightly closed with a muslin cloth and incubated under the above-mentioned conditions. Next, the female ticks were incubated for 15 days, and the mortality rate of female ticks was recorded. After oviposition, the eggs were collected, weighed, and stored under similar conditions till hatching. After 15 days, the tubes were checked to observe the hatched larvae in both treatment and control samples to estimate the hatching rates. The egg productive index (EPI) and hatchability of the laid eggs were calculated as follows:
EPI = egg mass weight (mg)/initial weight of engorged females (mg) (Alves et al., 2017; Abuowarda et al., 2020).
Percentage of hatchability = Number of hatched larvae/number of laid eggs × 100 (Abuowarda et al., 2020).
Preparation of tick specimens for histopathology
A total of 25 pairs of unfed females and males were fed on rabbits and detached after 7 days. The 25 semifed females were divided into five equal groups for histopathological examination. The 1st, 2nd, 3rd, and 4thgroups were immersed in 9.5% petroleum ether extract of M. azedarach, 28.6% ethanol extract of M. azedarach, 8% petroleum ether extract of A. herba-alba, and 38.6% ethanol extract of A. herba-alba, respectively for 1 min. These concentrations were chosen based on being the most effective on the engorged females and may have the ability to cause obvious damage in the tissues of semifed females that close to the engorged females in response to the extracts. The 5th group was left without treatment as a control. After 24 h, the tick samples were fixed in 10% formalin and then processed according to the method described by Agyei and Runham (1995). The tick sections were deparaffinized and stained with hematoxylin and eosin (H&E) for histological examination by light microscopy. The cuticle and gut of ticks were examined and photographed using an Olympus CX41 microscope.
Statistical analyses
Data were statistically evaluated by one-way ANOVA, followed by Tukey’s test, via the SPSS program version 20. The lethal concentration (LC50) values were determined by using a regression equation analysis to the probit transformed data of mortality. The dose response data were analyzed by the probit method (Finney, 1962).
Results
GC-MS analysis
A total of 20 and 10 compounds were distinguished in M. azedarach and A. herba-alba, respectively, using the GC-MS library (Tables 1 and 2). The major compounds present in M. azedarach extract belonged to the oil group, and several compounds belonged to the groups of organ-halogen, flavonoids, and terpenoids (a compound for each group). The compounds present in A. herba-alba belonged to the phytochemical groups, viz., terpenoids (2), oil (2), phenolic (2), monoterpenoids (1), limonoids (1), vitamin derivatives (1), and food additives (1).
Effect of the plant extracts on larvae
All extracts exhibited acaricidal effects against H. dromedarii larvae (Table 3). In both M. azedarach and A. herba-alba, petroleum ether extracts had more potential acaricidal activity than ethyl alcohol extracts. The most effective extract on larvae was the petroleum ether extract of A. herba-alba, which resulted in 100% mortality at 8% and 4% concentrations. All extracts resulted significantly lower mortalities than the reference acaricide (Butox®5.0), except the two higher concentrations of A. herba-alba petroleum ether extract that resulted in complete mortality similar to that recorded with Butox®5.0 treatment. In general, no mortality was recorded in the control groups.
Effect of the plant extracts on unfed adults
During the 2 days after treatment, it was observed that the treated ticks moved slowly, very few numbers of ticks were
Table 1: Gas chromatography-mass spectrometry of petroleum ether extract of Melia azedarach using library.
| Phytochemical group | Area% | Name | RT |
|
oil |
1.84 | Dibutyl ester of decanedioic acid | 7.44 |
| Oil | 1.76 | 1-Dodecene, 2-ethyl | 9.83 |
| Oil | 3.88 | (E)-2,3-Epoxy-1-(meth oxymethoxy)octane | 10.02 |
| Oil | 2.14 | Nonacosane | 10.48 |
| Organ halogen | 3.05 | Russuphelol | 11.32 |
| oil | 6.24 | 6-Hydroxy-2-hexanone | 15.67 |
| oil | 1.87 | 2-Decenal, (E)- | 16.03 |
|
oil |
2.68 | Cyclohexanecarboxylic acid, | 16.22 |
| flavonoids | 1.52 | Flavomannin 6,6',8-tri-O-methyl ether | 16.52 |
| oil | 4.24 | 1-Butyne, 3,3-dimethyl( | 16.93 |
| oil | 7.26 | 2,4-Decadiena | 17.54 |
| oil | 5.73 | 3-Hexene | 18.34 |
| oil | 14.81 | 4-Nonanone, 7-ethyl- | 18.72 |
| oil | 1.04 | 9,11-Dodecadien-1-ol | 19.92 |
| Terpenoids | 1.52 |
α - sesquiterpenol |
22.31 |
| oil | 2.20 | Hexadecanoic acid, methyl ester | 31.14 |
| oil | 6.35 | 1- Nonynoic acid, methyl ester | 34.30 |
| oil | 2.51 | 9-Dodecenoic acid, methyl ester | 34.39 |
| oil | 1.32 | Eicosane | 37.74 |
| oil | 0.54 | 1-Heptadecene |
38.94 |
RT: Retention Time
Table 2: Gas chromatography-mass spectrometry of petroleum ether extract of Artemisia herba-alba using library.
| Phytochemical group | %Area | Name | RT |
| terpenoids | 1.17 | Camphor | 12.23 |
| oil | 21.2 | 1-Hexen-3-yne, 2,5,5-trimethyl | 17.87 |
| Phenolic | 4.16 | Ethyl (E)-Cinnamate | 21.45 |
| Vitamin derivative | 1.74 | (-)-3-alpha Acetoxy-5-etienic acid | 24.07 |
| terpenoids | 3.77 | Bicyclo[2.2.1]heptan-2one,4,7,7-trimethyl-, semicarbazone | 27.49 |
| oil | 22.98 | TRICYCLO[6,8,9)]HEXADECA-3,16-Dion | 35.61 |
| Food additive | 6.73 | Dihydrocarvyl acetate | 35.80 |
| Mono-terpenoids | 0.63 | chrysanthenone | 36.13 |
| limonoids | 0.37 | R-Limonene | 36.37 |
| Phenolic | 0.73 | Hydrocinnamic acid |
37.45 |
RT: Retention Time
Table 3: Mortality percentages (mean ± SE) of Hyalomma dromedarii larvae treated with petroleum ether and ethyl alcohol extracts of Melia azedarach and Artemisia herba-alba at 24 h post treatment.
| Melia azedarach | Artemisia herba-alba | ||||||
| Petroleum ether | Ethyl alcohol | Petroleum ether | Ethyl alcohol | ||||
|
Conc. (%) |
Mortality (%) |
Conc. (%) |
Mortality (%) |
Conc. (%) |
Mortality (%) |
Conc. (%) |
Mortality (%) |
| 19.1 |
69.73±6.64c |
28.6 |
56.3±14.66b |
8 |
100±0.00c |
38.6 |
67.60±5.73d |
| 9.6 |
43.93±10.60b |
14.3 |
28.62±3.62ab |
4 |
100±0.00c |
19.3 |
52.08±14.58cd |
| 4.8 |
19.99±0.61a |
7.15 |
6.64±1.65a |
2 |
48.43±15.91b |
9.65 |
29.72±1.84bc |
| 2.4 |
19.45±3.40a |
3.5 |
5.56±2.17a |
1 |
18.33±1.66ab |
4.82 |
20.20±3.06ab |
|
Butox®5.0 (1ml/L) |
100±0.00d |
Butox®5.0 (1 ml/L) |
100±0.00c |
Butox®5.0 (1 ml/L) |
100±0.00c |
Butox®5.0 (1 ml/L) |
100±0.00 e |
| Control |
0.00±0.00a |
Control |
0.00±0.00 a |
Control |
0.00±0.00a |
Control |
0.00±0.00a |
| F value | 62.14 | F value | 21.06 | F value | 48.08 | F value | 53.09 |
| P value | <0.001 | P value | <0.001 | P value | <0.001 | P value |
<0.001 |
a, b…… etc, indicate the significant difference between the mean values of mortality percentages according to Tukey’s test.
Conc: Concentrations.
Table 4: Mortality percentages (mean±SE) of Hyalomma dromedarii unfed adults treated with petroleum ether and ethyl alcohol extracts of Melia azedarach and Artemisia herba-alba at 3 days post treatment.
| Melia azedarach | Artemisia herba-alba | ||||||
| Petroleum ether | Ethyl alcohol | Petroleum ether | Ethyl alcohol | ||||
|
Conc. (%) |
Mortality (%) |
Conc. (%) |
Mortality (%) |
Conc. (%) |
Mortality (%) |
Conc. (%) |
Mortality (%) |
| 38.2 |
90±10.0cd |
28.6 |
56.66±6.66c |
16 | 100±0.00d | 38.6 |
32.5±6.29c |
| 19.1 |
90±10.0cd |
14.3 |
46.66±8.81bc |
8 |
80±0.10.0cd |
19.3 |
20.00±0.00bc |
| 9.6 |
63.3±3.33bc |
7.15 |
26.6±8.81ab |
4 |
66±11.54c |
9.65 |
16.66±3.33abc |
| 4.8 |
46.6±6.66b |
3.57 |
13.3±3.33a |
2 |
36±11.54b |
4.82 |
6.66±3.33ab |
|
Butox®5.0 (1ml/L) |
100±0.00d |
Butox®5.0 (1 ml/L) |
100±0.00d |
Butox®5.0 (1 ml/L) |
100±0.00d |
Butox®5.0 (1 ml/L) |
100±0.00d |
| Control |
0.00±0.00a |
Control |
0.00±0.00a |
Control |
0.00±0.00a |
Control |
0.00±0.00a |
| F value | 29.23 | F value | 36.46 | F value | 75.58 | F value | 78.082 |
| P value | <0.001 | P value | <0.001 | P value | <0.001 | P value | <0.001 |
a, b…… etc, indicate the significant difference between the mean values of mortality percentages according to Tukey’s test.
Conc: Concentrations.
Table 5: Mortality percentages (mean±SE) of Hyalomma dromedarii females treated with petroleum ether and ethyl alcohol extracts of Melia azedarach and Artemisia herba-alba.
|
Melia azedarach |
Artemisia herba-alba |
|||||||
|
Petroleum ether |
Ethyl alcohol |
Petroleum ether |
Ethyl alcohol |
|||||
|
Conc. (%) |
Mortality (%) |
Conc. (%) |
Mortality (%) |
Conc. (%) |
Mortality (%) |
Conc. (%) |
Mortality (%) |
|
|
9.5 |
90±10.00d |
28.6 |
85±5.00d |
8 |
90±10.00d |
38.6 |
50±10.00c |
|
|
4.7 |
60±0.00c |
14.3 |
60±0.00 c |
4 |
60±0.00 c |
19.3 |
40±0.00bc |
|
|
2.3 |
20±0.00a |
7.1 |
26.6±13.33b |
2 |
20±0.00 ab |
9.6 |
20±11.5ab |
|
|
1.1 |
6.6±6.66a |
3.5 |
0.00±0.00 a |
1 |
6.66±6.66 a |
4.8 |
0.00±0.00 a |
|
|
Butox®5.0 (1ml/L) |
33.33±6.66b |
Butox®5.0 (1 ml/L) |
33.33±6.66b |
Butox®5.0 (1 ml/L) |
33.33±6.66b |
Butox®5.0 (1 ml/L) |
33.33±6.66bc |
|
|
Control |
0.00±0.00a |
Control |
0.00±0.00a |
Control |
0.00±0.00 a |
Control |
0.00±0.00 a |
|
|
F value |
27.78 |
F value |
27.64 |
F value |
27.78 |
F value |
7.38 |
|
|
P value |
<0.001 |
P value |
<0.001 |
P value |
<0.001 |
P value |
<0.001 |
|
a, b…… etc, indicate the significant difference between the mean values of mortality percentages according to Tukey’s test.
Conc: Concentrations.
dead, and the majority of ticks were dead on the 3rd day post treatment (Table 4). The petroleum ether extract of A. herba-alba was the most effective against unfed adults similar to that recorded in larvae. This extract resulted in 100% mortality at 16% concentration similar to that recorded with Butox®5.0 treatment. The two highest concentrations
38.2% and 19.1% of M. azedarach petroleum ether extract resulted in 90% ±10.0% mortality, which was non-significantly lower than that recorded with Butox®5.0 treatment. The control groups showed no mortality.
Table 6: Reproductive performance of Hyalomma dromedarii females treated with petroleum ether and ethyl alcohol extracts of Melia azedarach.
| Petroleum ether | Ethyl alcohol | ||||||
|
Conc. (%) |
EPI | Egg number |
Hatchability (%) |
Conc. (%) |
EPI | Egg number |
Hatchability (%) |
| 9.5 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 28.6 |
0.183±0.00ab |
976.19±0.00ab |
84.42±0.00b |
| 4.7 |
0.069±0.00a |
601.90±0.00a |
99.98±0.00b |
14.3 |
0.261±0.097b |
1780.00±576.8bc |
89.62±7.24b |
| 2.3 |
0.101±.0143a |
680.90±55.04a |
99.99±0.010b |
7.1 |
0.395±0.020c |
2634.3±145.1cd |
97.17±0.423b |
| 1.1 |
0.2900±0.052b |
2393.65±393.7b |
99.98±0.0103b |
3.5 |
0.533±0.032d |
3514.28±140.52d |
98.03±0.569b |
|
Butox®5.0 (1ml/L) |
0.15±0.017a |
623.7±226.3a |
65.9±12.4a |
Butox®5.0 (1 ml/L) |
0.15±0.017a |
623.7±226.3a |
65.9±12.4a |
| Control |
0.528±.0.029c |
3761.7±281.3c |
99.12±0.115b |
Control |
0.621±.011d |
5287.7±359.3e |
99.5±0.16b |
| F value | 25.65 | 23.79 | 6.70 | F value | 43.87 | 45.34 | 6.47 |
| P value |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
a, b…… etc, indicate the significant difference between the mean values of each parameter according to Tukey’s test.
EPI: Egg Productive Index.
Conc: Concentrations.
Table 7: Reproductive performance of Hyalomma dromedarii females treated with petroleum ether and ethyl alcohol extracts of Artemisia herba-alba.
| Petroleum ether | Ethyl alcohol | ||||||
|
Conc. (%) |
EPI | Egg number |
Hatchability (%) |
Conc. (%) |
EPI | Egg number |
Hatchability (%) |
|
8 |
0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 38.6 |
0.230±0.055ab |
1020.76±105.38a |
29.98±18.96a |
| 4 |
0.432±.0033bc |
4493.22±363.8a |
79.83±11.50ab |
19.3 |
0.44±0.035c |
2497.41±537.9b |
90.65±1.89c |
| 2 |
0.522±0.048c |
4459.8±373.69a |
78.46±7.033ab |
9.6 |
0.28±0.05b |
2088.09±457.44b |
97.5±0.02c |
| 1 |
0.351±0.042b |
3002.75±343.65a |
80.12±7.65ab |
4.8 |
0.47±0.033c |
2606.34±200.3b |
95.95±0.99c |
|
Butox®5.0 (1ml/L) |
0.15±0.017a |
623.7±226.3a |
65.9±12.4a |
Butox®5.0 (1 ml/L) |
0.15±0.017a |
623.7±226.3a |
65.9±12.4b |
| Control |
0.528±.0.029c |
3761.7±281.3a |
99.12±0.115b |
Control |
0.62±.011d |
5287.7±359.3c |
99.5±0.16c |
| F value | 16.82 | 0.808 | 2.499 | F value | 25.75 | 25.62 | 13.52 |
|
P value |
<0.001 |
<0.525 |
<0.053 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
a, b…… etc, indicate the significant difference between the mean values of each parameter according to Tukey’s test.
EPI: Egg Productive Index.
Conc: Concentrations,
Effect of the plant extracts on engorged females
The two highest concentrations of petroleum ether extract of both M. azedarach (4.7% and 9.5%) and A. herba-alba (4% and 8%) resulted significantly higher mortalities (60%–90%) than that recorded with Butox®5.0 treatment (33.3%) (Table 5). Moreover, the two highest concentrations of M. azedarach ethyl alcohol extract (14.3% and 28.6%) resulted significant mortalities of 60% and 85%, respectively, compared to that recorded with Butox®5.0 treatment (33.3%). No mortality was recorded in the control groups.
Comparative acaricidal effect of the plant extracts on the active stages of ticks
The LC50 values confirmed that the petroleum ether extracts were more toxic against the active stages of H. dromedarii than the ethyl alcohol extracts of the two tested plants (Figure 1). Moreover, the petroleum ether extract of A. herba-alba was more toxic than that of M. azedarach against all stages. In addition, the LC50 values indicated that the petroleum ether extract of A. herba-alba was more toxic against larvae (1.83%), followed by unfed adults (2.378%) and then engorged females (3.1%). However, the petroleum ether extract of M. azedarach exhibited its activity in the reverse order, i.e., it was more toxic against engorged females (3.969%), followed by unfed adults (5.473%), and then larvae 10.99%. The LC50 values of the ethyl alcohol extracts of A. herba-alba and M. azedarach for larvae, unfed adults, and engorged females were 19.60% and 22.75%, 79.812% and 19.443%, and 29.631% and 11.13%, respectively. These LC50 values indicated that the toxicity of ethyl alcohol extracts of the two plants against larvae appeared to be similar to each other; however, the ethyl alcohol extract of M. azedarach was more toxic against unfed adults and engorged females than the ethyl alcohol extract of A. herba-alba.
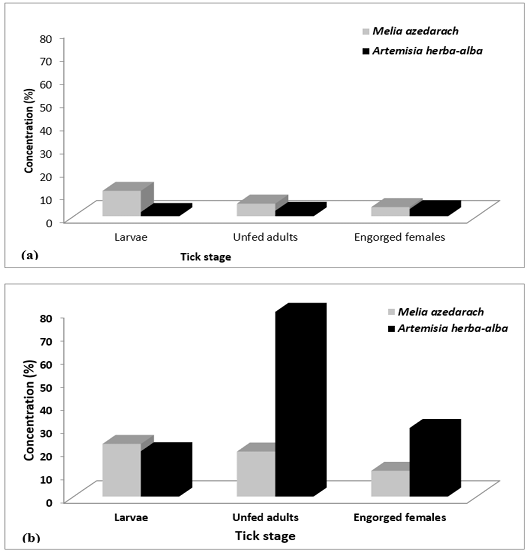
Figure 1: Lethal concentrations of 50% (LC50) for larvae, unfed adults, and engorged females of H. dromedarii treated with plant extracts prepared from M. azedarach and A. herba-alba, (a) petroleum ether extracts, (b) ethyl alcohol extracts.
Effect of the plant extracts on the reproductive performance of engorged females
Tables 6–7 show the reproductive performance, including EPI, egg number, and hatchability percentage, of H. dromedarii engorged females exposed to the petroleum ether and ethyl alcohol extracts of M. azedarach and A. herba-alba for those females that succeeded till the completion of life and laid eggs. EPI increased with the decrease in concentration of both petroleum ether and ethyl alcohol extracts of M. azedarach, whereas it was higher for the two extracts of A. herba-alba, especially at the lowest concentration of each extract (Table 7). The EPI achieved by treatment with the petroleum ether extract of M. azedarach ranged from 0.069±0.00 to 0.2900±0.052 compared to the EPI of 0.15±0.017 recorded with Butox®5.0 treatment and 0.528±.0.029 in the control group. For the A. herba-alba petroleum ether extract treatment, the EPI ranged from 0.0 to 0.522±0.048. With the ethyl alcohol extract treatment of M. azedarach, the EPI ranged from 0.183±0.00 to 0.533±0.032 compared to 0.15±0.017 recorded with Butox®5.0 treatment and 0.621±0.011 in the control group. For the A. herba-alba ethyl alcohol extract treatment, the EPI ranged from 0.230±0.055 to 0.47±0.033. In general, the treatments resulted in high EPI and high egg numbers (Tables 6–7). The egg numbers were 0.0 to 2393.65±393.7, 0.0 to 4459.8±373.69, 976.19±0.00 to 3514.28±140.52, and 1020.76±105.38 under treatments with the petroleum extract of M. azedarach, petroleum extract of A. herba-alba, ethyl alcohol extract of M. azedarach, and ethyl alcohol extract of A. herba-alba, respectively, compared to 623.7±226.3 recorded with Butox®5.0 treatment, 3761.7±281.3 recorded in the petroleum ether control group, and 5287.7±359.3 recorded in the ethyl alcohol control group. Most of the eggs laid by the treated females succeeded to hatch as recorded by the hatchability percentages, ranging from 29.98% ±18.96% to 99.99% ±0.010% compared to 65.9% ±12.4% recorded with Butox®5.0 treatment, 99.12% ±0.115% recorded in the petroleum ether control group, and 99.5% ±0.16% in the ethyl alcohol control group (Tables 6–7). The eggs laid by the females treated with the petroleum ether extract of M. azedarach achieved a high percentage of hatchability (>99%) similar to that in the control group. However, the petroleum ether extract of A. herba-alba resulted in a lower hatchability percentage (<81%) than that in the control group. Moreover, the hatchability percentage under treatment with M. azedarach ethyl alcohol extract was high in the range of 84.42%–98.03%. The ethyl alcohol extract of A. herba-alba also resulted in a high hatchability percentage, ranging from 90.65% ±1% to 97.5% ±0.02%.
Histopathological examination of semifed females treated with the plant extracts
The histological sections of the partially fed females of H. dromedarii in the control group showed that the integument is formed of a cuticle and an epithelial cell layer supported by a basal lamina. The cuticle is split into two major regions, an outer thinner and slightly folded layer (zigzag-like) known as epicuticle and an inner thicker layer termed procuticle. This procuticle has two well-defined parts, the exocuticle (adjacent to the epicuticle) and the endocuticle (adjacent to the epithelial layer). The epithelium is formed by a monolayer of epithelial cells with their nuclei (Figure 2a). The midgut epithelial cells are stratified epithelium with nuclei generally spherical and less vacuolated cytoplasm. The epithelial wall of the gut is supported with thin basal membrane and thin layer of muscular tissue. It appeared normal without any damage and well adhesive to the gut wall beside each other (Figure 2b).
The partially fed females of H. dromedarii treated with the petroleum ether and ethyl alcohol extracts of M. azedarach and A. herba-alba observed alterations such as the epicuticle and procuticle with less thickness compared to those in the control group, and the epicuticle was also less folded than that in the control group (Figure 2c). There was also complete degeneration of the epithelial cells lining the gut (Figure 2d). The partially fed females treated with the ethyl alcohol extract of M. azedarach showed thinning in the cuticle with slight separation of the epithelial layer (Figure 2e). The gut epithelium also exhibited slight degeneration (Figure 2f). The cuticle of the partially fed females treated with A. herba-alba petroleum ether extract exhibited severe separation of the epithelial layer (Figure 2g), and the gut showed severe damage of epithelial cells and was detached inside the gut lumen (Figure 2h). The effect of the ethyl alcohol extract of A. herba-alba did not produce significant alterations compared to those in the control group. The cuticle and midgut characteristics were preserved, except slight separation of the epithelial layer (Figure 2i) and slight degeneration of epithelial cells of the gut (Figure 2j).
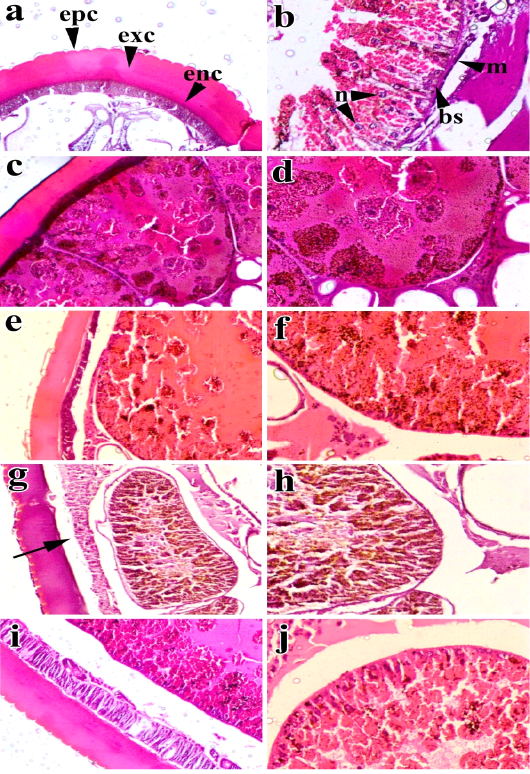
Figure 2: Cross sections of semifed females of H. dromedarii treated with four plant extracts: a-b: untreated (control), c-d: treated with petroleum ether extract of M. azedarach, e-f: treated with ethyl alcohol extract of M. azedarach, g-h: treated with petroleum ether extract of A. herba-alba, i-j: treated with ethyl alcohol extract of A. herba-alba. The images (a), (c), (e), (g), and (i) photographed at 10× indicate the changes in the cuticles. The images (b), (d), (f), (h), and (j) photographed at 20× indicate the changes in gut walls and their epithelial cells. epc: epicuticle, exc: exocuticle, enc: endocuticle, m: muscle layer, bs: basal membrane, n: nucleus.basal
Discussion
Although M. azedarach and Artemisia spp. plants possess antiparasitic properties, only a few studies have evaluated their acaricidal activities on ticks. The extracts of M. azedarach were found to exhibit acaricidal effects on different developmental stages of the tick Rhipicephalus microplus (Borges et al., 2003; De Sousa et al., 2014). Similarly, the extracts of Artemisia spp. were found to exert acaricidal activity against various stages of tick species, such as A. abrotanum against the tick Ixodes ricinus (Tunon et al., 2006), A. annua against the tick R. microplus (Chagas et al., 2011), and A. absinthium against the ticks R. sanguineus and Hyalomma anatolicum (Godara et al., 2014; 2015). Moreover, studies have reported the larvicidal and repellent effects of A. herba-alba against H. dromedarii larvae and I. ricinus nymphs, respectively (Abdel-Shafy et al., 2007; El-Seedi et al., 2017). In addition, the susceptibility of the developmental stages of H. dromedarii to the natural products extracted from M. azedarach and A. herba-alba was not evaluated, except H. dromedarii larvae that were confirmed to be sensitive to A. herba-alba extracts (Abdel-Shafy et al., 2007). In our previous study, the petroleum ether and ethyl alcohol extracts of M. azedarach and A. herba-alba exhibited strong acaricidal activity against the inactive stages (off host) such as embryonated eggs and engorged nymphs of H. dromedarii (Abdel-Ghany et al., 2019). Sustainable tick control must be on and off the host (Godara et al., 2015). Therefore, the present study evaluated the effects of the petroleum ether and ethyl alcohol extracts of M. azedarach and A. herba-alba against the active developmental stages (on host), such as larvae, unfed adults, and engorged females, of the camel tick H. dromedarii under laboratory conditions.
In the present study, the larvae responded to the plant extracts more rapidly than the unfed adults that moved slowly during the 2 days’ post treatment and were dead on the 3rd day, whereas mortality was recorded at 24 h post treatment in the larvae. This finding may be attributed to large chitinized cuticle area of unfed adults compared less chitinized area of the body of the larvae. The nonpolar petroleum ether extracts of the two plants M. azedarach and A. herba-alba were more toxic than the ethyl alcohol extract against the larvae, unfed adults, and engorged females. This finding may be attributed to the active constituents that may have more potential acaricidal effects than those in the ethyl alcohol extracts. The results of GC-MS analyses in the present study showed that M. azedarach petroleum ether extract contained 20 major constituents belonging to the oil group, flavonoids, and terpenoids. This finding is in agreement with the results of Borges et al. (2003) who reported that the activity of M. azedarach fruit extracts prepared using nonpolar and intermediate-polarity solvents was due to the presence of steroids and terpenoids. Moreover, Carpinella et al. (2007) demonstrated the highly toxic effect of M. azedarach on head lice and detachment of eggs from hair due to the presence of the highly oil constituents of this plant. Moreover, the petroleum ether extract of A. herba-alba contains 10 compounds, and the major chemical constituents were terpenoids, oils, phenolics, monoterpenoids, and limonoids. Most of the previous studies have attributed the insecticidal effect of A. herba-alba to its essential oil constituents (Soliman, 2006; Bachrouch et al., 2015; Badreddine and Baouindi, 2016; Bouzeraa et al., 2018). The mortality observed in relation to the toxic effect of the oil is due to volatile oil substances that might block the tracheal respiration of the insects, leading to their death (Pugazhvendan et al., 2012). Several studies have shown that most of the essential oils are composed of monoterpene compounds that bind to protein receptors and act by inhibiting the enzyme acetylcholinesterase (Chaubey, 2014; 2017), interfering the neuromodulator octopamine (Enan, 2005) or GABA-gated chloride channels (Priestley et al., 2006), and interrupting neurotransmission, leading to physiological imbalance and thereafter the death of the target insect.
On the other hand, the ethanol extracts of the two plants exhibited weaker effects on all the active tick stages. This finding may be attributed to the lower percentages of secondary metabolite chemicals in the extracts. When compared with the reference acaricide, all extracts resulted in lower mortality in larvae and unfed adults, except the two highest concentrations of A. herba-alba petroleum ether extract that resulted in complete mortalities similar to that with Butox®5.0 treatment. However, the reference acaricide demonstrated low toxicity against engorged females.
The calculated LC50 values confirmed that the petroleum ether extract of A. herba-alba was more toxic against larvae, followed by unfed adults and then engorged females, and the petroleum ether extract of M. azedarach was more lethal against engorged females, then unfed adults and then larvae. The ethyl alcohol extracts of the two plants showed the same response against larvae. The ethyl alcohol extract of M. azedarach was more toxic against unfed adults and engorged females than that of A. herba-alba. In contrast to the results of this study, our previous study (Abdel-Ghany et al., 2019) demonstrated that the petroleum ether extracts of both M. azedarach and A. herba-alba exhibited lower toxicity against H. dromedarii eggs (LC50: 3.14% and 3.91%) than the ethyl alcohol extracts (1.77% and 2.45%). However, the previous study results were in agreement with those of the present study, as the petroleum ether extracts of the two medicinal plants were more toxic against the engorged nymphs of H. dromedarii (LC50: 0.26% and 1%) than the ethyl alcohol extracts (LC50: 4.17% and 8.7%). The essential oil of A. herba-alba was found to exhibit a repellent activity of 84.2% on I. ricinus (El-Seedi et al., 2017). This repellent activity was attributed to the major chemical constituents of the essential oil, which contained piperitone, ethyl cinnamate, camphor, and hexadecanoic acid. Consistent with the present study, Godara et al. (2014 and 2015) confirmed the acaricidal effect of chloroform and ethyl alcohol extracts of A. absinthium against the adults and larvae of both R. sanguineus and H. anatolicum, respectively. They reported that the two extracts resulted in 100% mortality in the larvae after 24 h, and the LC50 values were 8.8% and 6.5% in the adults. However, Parveen et al. (2014) found that the ethyl alcohol extract was less toxic against R. microplus adults, with the LC50 value being 11.2%. This finding may be attributed to the difference in the environmental condition of the geographical locations where the plant grew or the sensitivity of the tick species to the plant extract.
In the present study, the EPI and egg number of engorged females treated with the plant extracts (M. azedarach and A. herba-alba) and the reference acaricide (Butox®5.0) were approximately lower than those in the control group. However, the hatchability percentage under treatment with the plant extracts was higher than that under treatment with the reference acaricide and approximately similar to that in the control group. The significant reductions in EPI and egg numbers indicated that the two extracts of M. azedarach were more effective than those of A. herba-alba. This implies that M. azedarach extracts have more potential extended effects on H. dromedarii engorged females. In agreement with the present study results, some studies have reported that extracts prepared from M. azedarach and Artemisia spp. exhibited similar action on the engorged females of various tick species, with some differences in hatchability. Borges et al. (2003) reported that M. azedarach extracts inhibited partially or totally the eggs laid by treated fully fed females. De Sousa et al. (2014) showed that the hexane extract of M. azedarach un-ripened fruits could reduce the reproductive capacity of R. (B.) microplus. Reductions in oviposition and hatchability were reported in the dog tick R. sanguineus exposed to the chloroform extract of A. absinthium (Godara et al., 2014) and in the ticks R. microplus and H. anatolicum exposed to the ethanol extract of A. absinthium (Parveen et al., 2014; Godara et al., 2015). However, R. annulatus females exposed to 60 μL/cm3 essential oil of A. annua failed to lay eggs (Pirali-Kheirabadi and Teixeira da Silva, 2011). In the present study, partially fed females of H. dromedarii treated with the petroleum ether and ethyl alcohol extracts of M. azedarach and A. herba-alba suffered from various changes, especially in the cuticle and gut. According to our opinion, the selection of semifed females for this test based on this stage is a better model for examining the external and internal structures. This stage has a wide external area of cuticle that is lesser hard than that of unfed adults and has a gut filled with medium amount of host blood. The extracts of the two plants led to thinning and separation of epithelial cells, with destruction of epithelial cells lining the gut of partially fed females.
The histopathological effects of the petroleum ether and ethyl alcohol extracts of M. azedarach and A. herba-alba in the present study were consistent with the results mentioned by Remedio et al. (2014) who examined the effect of the aqueous extracts of neem leaves on R. sanguineus synganglion and integument. They found that the epithelial cells of the integument and nerve cells of the synganglion exhibited signs of cell vacuolation, cellular disorganization, and variations in subcuticle thickness.
Conclusion
The two plants M. azedarach and A. herba-alba exhibited variable acaricidal activities against the active stages of H. dromedarii. The petroleum ether extract of A. herba-alba was more toxic against larvae, followed by unfed adults and then engorged females. However, that of M. azedarach showed its activity in the reverse order. The effect of ethyl alcohol extracts of the two plants on larvae appeared to be similar to each other; however, the ethyl alcohol extract of M. azedarach was more toxic against unfed adults and engorged females than that of A. herba-alba. Therefore, these two plants might be incorporated in the elements of the integrated control programs for camel ticks. Further in vivo study required to evaluate the toxicity of these medicinal plants to be safely used in the treatment of the infested animals.
Acknowledgments
This study is a part of a PhD thesis to be submitted to the Department of Parasitology, Faculty of Veterinary Medicine, Cairo University. The study was carried out by financial support of National Research Centre as a part of PhD Thesis No. 12/2/19.
Authors’ Contributions
MMF, SA, MMA, RME, and HSMA designed the experiments. EMH and HSMA participated in the preparation of plant extracts. MMF, SA, MMA, RME, and HSMA participated in the following protocols: bioassay of the plant extracts against active stages of H. dromedarii such as larvae, unfed adults, and engorged females and detection of the histopathological effect of extracts on the cuticle and gut of treated ticks. SA and HSMA analyzed and tabulated the data. MMF, SA, MMA, and HSM wrote the draft of the manuscript. All authors revised and approved the final version of the manuscript.
Compliance with Ethical Standards
This study was approved by the Ethical Committee for Medical Research (MREC) at the National Research Centre (NRC), Egypt, in accordance with local laws and regulations (approval protocol No 20022). Consent was obtained from the owners of camels included in this study.
Conflict of Interest
The authors declare that there is no conflict of interest.
References






