Advances in Animal and Veterinary Sciences
Research Article
Prevalence and Predisposing Factors in Spontaneous Cases of Necrotic Enteritis in Cage Reared Commercial Layer Chicken
Perumal Balachandran1, Palani Srinivasan1*, Gurusamipalayam Amirthalingam Balasubramaniam1, Soundarapandiyan Sivaseelan1, Thippichettipalayam Ramasamy Gopala Krishna Murthy2
1Department of Veterinary Pathology, Veterinary College and Research Institute, Namakkal, India; 2Poultry Disease Diagnosis and Surveillance Laboratory, Veterinary College and Research Institute Campus, Namakkal, India.
Abstract | Necrotic enteritis (NE) caused by C. perfringens is one of the economically important disease in broiler chicken. Nowadays frequent outbreak were noticed in commercial layer flocks maintained in raised cage system and causing mortality and decreased egg production. Hence the present study was undertaken to assess the prevalence and predisposing factors associated with spontaneous cases of necrotic enteritis in cage reared commercial layer chicken. A total of 1000 white leghorn carcasses, above 20 weeks (wk) age from 100 flocks with gastrointestinal tract disorders were screened for the presence of necrotic enteritis lesions and C. perfringens out of which 28 flocks (28%) showed lesions and organism. All the 28 isolates were identified as alpha toxin producing strains by PCR technique. Affected flocks showed depression, soiling of vent with brownish fecal materials and sudden death. Necropsy revealed dilated intestine and Turkish towel appearance of mucosa due to necrosis caused by alpha toxin of C. perfringens. Necrotic enteritis was recorded as single (13 %) and combined (15%) infection with coccidiosis (11%), worm infestation (2%) and Newcastle disease (2%). Highest prevalence was noticed in the age group of 21-30 wk (42.86%) and Northeast monsoon season (60.71%). Drop in egg production and mortality in the affected flocks was 3.0 to 10 and 2. 72 to 3.15 % respectively. Incidence of disease was more in flocks which used animal protein as a source of feed ingredient.
Keywords | Layer chicken, Necrotic enteritis, Prevalence, C. perfringens, Polymerase chain reaction (PCR).
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | February 09, 2017; Accepted | March 13, 2018; Published | March 25, 2018
*Correspondence | Palani Srinivasan, Veterinary College and Research Institute Campus, Namakkal, India; Email: [email protected]
Citation | Balachandran P, Srinivasan P, Balasubramaniam GA, Sivaseelan S, Murthy TRGK (2018). Prevalence and predisposing factors in spontaneous cases of necrotic enteritis in cage reared commercial layer chicken. Adv. Anim. Vet. Sci. 6(3): 113-120.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.3.113.120
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Balachandran et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Poultry is one of the fastest growing and profitable segments of the agricultural sector in India with around eight percent growth rate per annum. Development of high yielding layer (310- 340 eggs) strains together with standardized package of practices on nutrition, housing, management and disease control have contributed to spectacular growth rates in egg production (4-6% per annum) in India during the last 40 years. Among the various region of India, southern, eastern and central and northern and western regions respectively account for about 57, 17 and 26 percent of egg production. Among the southern states Telengana and Andhra Pradesh together contributes 50 % , Tamil Nadu contributes 30% and the remaining 20% is contributed by Karnataka and Kerela (Chatterjee and Rajkumar, 2015). In Tamil Nadu, Namakkal district have a layer population of 4.5 crores and producing around 3.5 crore eggs per day. (Srinivasan et al., 2012).
One of the most critical concerns of poultry production is that the birds should have healthy and functional gastrointestinal tract to avoid production loss, increased mortality, and risk of contamination of poultry products (Porter, 1998). Physical, chemical and biological disturbances in gastrointestinal tract can result in enteric diseases. Among the enteric diseases of poultry necrotic enteritis caused by Clostridium perfringens is an acute or subacute enterotoxemia (Cooper et al., 2013).
Even though the NE is a disease of broiler birds nowadays frequent outbreak were noticed in commercial layer flocks maintained in raised cage system of management and causing mortality and decreased egg production. Prevalence of necrotic enteritis is influenced by many factors like rearing, season, age of birds and concurrent enteric infections (Craven et al., 2001). Risk factors for necrotic enteritis have mostly been investigated using experimental studies (Sivaseelan et al., 2013) and few field based surveys (Sawale et al., 2010; Malmurugan et al., 2012). Hence the present paper enlightens the prevalence and the risk factors associated with the spontaneous cases of necrotic enteritis in cage reared commercial layer flocks in Namakkal region of Tamil Nadu, India.
Materials and Methods
Data Collection
The study was carried out over a period of 3 years (October 2008 to September 2011). The prevalence and predisposing factors of necrotic enteritis in commercial layer chickens in and around Namakkal district was studied in 100 flocks above 20 wk with the history, symptoms and postmortem lesions suggestive of gastrointestinal tract disorders. Information regarding breed and strain of chicken, flock strength, age, method of rearing, raw materials used for manufacturing of compound feed, vaccination schedule, production performance, symptoms and mortality were collected.
Necropsy Examination
A total of 1000 dead birds from 100 selected flocks (10 per flock) were subjected to detailed postmortem examination as per approved procedure. Gastrointestinal tract was examined systematically for gross pathological changes. Intestinal scrapping collected from the selected flocks were screened after staining with Grams stain by direct microscopic examination. During postmortem examination, concurrent infections were diagnosed based on the gross lesions and laboratory tests.
Bacterial Isolation
For isolation of the organism, causing necrotic enteritis, pooled intestinal contents and scrapping were collected from those flocks which are showing gross lesions suggestive of necrotic enteritis and Clostridium organism on direct microscopic examination. Sterile saline was added to the collected materials and heated at 80oC for 20 min in water bath. Then the processed intestinal contents were inoculated into thioglycollate broth, Robertson cooked meat medium with brain heart infusion broth and sterile liquid paraffin was poured to make a layer over the medium. Inoculated medium was incubated anaerobically at 37oC for 24h. The presence of C. perfringens in the inoculated sample is indicated by turbidity in both the media. The positive cultures were streaked on to clostridial agar and perfringens agar with supplements. The plates were incubated in the anaerobic jar at 37oC for 48h. The plates were observed for the growth of characteristic colonies of C. perfringens (Barrow and Feltham, 1993). The feed, fishmeal and meat and bone meal were screened for the presence of C. perfringens as per the method described by Srinivasan et al. (2014).
Identification
The positive colonies obtained on clostridial and perfringens agar were inoculated on milk medium with reducing agent, egg yolk agar and sheep blood agar and they were gram stained and tested for their ability to ferment glucose, maltose, lactose, sucrose, and mannitol. The isolates were also subjected to oxidase, catalase, and gelatin liquification tests as described by Barrow and Feltham (1993).
Identification of Toxigenic Strains by Polymerase Chain Reaction (PCR)
Polymerase chain reaction of DNA extracted from C. perfringens isolates were performed according to the protocol of Baums et al. (2004). The alpha toxin specific oligonucleotide primers (M/s. Genei, India) used in this study were CP – F- AGT CTA CGC TTG GGA TGG AA and CP – R- TTT CCT GGG TTG TCC ATT TC which flanked 775 base pair DNA sequence. The PCR assay was performed in 50 µl reaction volume in thermal cycler. The reaction mixture consisted of 2 µl template DNA prepared by the heat lysis method, 0.5 µl of each forward and reverse primers, 21 µl of master mix (M/s Eppendorf, Germany) and 26 µl of molecular biology grade water. The PCR program was as follows: initial denaturation at 95oC for 2 min followed by 35 cycles of denaturation at 95oC for 1 min, annealing at 55oC for 1 min, extension at 72oC for 1 min 20 sec and followed by final extension at 72oC for 2 min. Six microlitre of the amplicons was separated by electrophoresis in a 1.5% agarose gel according to standard procedure. Bands were visualized by ethidium bromide staining.
Statistical Analysis
The data on age and month wise occurrence of necrotic enteritis were collected. The seasons were classified as summer (March, April and May), south west monsoon (June, July and August), north east monsoon (September, October and November) and winter (December, January and February). The age was classified into six groups viz., 21- 30, 31-40, 41-50, 51-60, 61-70 and 71-80 wk. Data on age, temperature, rainfall, season, egg production and mortality has been entered in to an excel spread sheet and transferr-
Table 1: Prevalence of Necrotic Enteritis in commercial layer chicken
| Flock No. | Age (Wk) | Flock Size | Drop in egg production (%) | Mortality (%) | Positivity of C. perfringens | Diagnosis | |||
| Fish meal | MBM | Feed samples | Intestinal contents | ||||||
| 1 | 55 | 20000 | 3.0 | 2.25 | + | + | + | + | NE |
| 2 | 23 | 4500 | 7.0 | 3.90 | + | + | + | + | NE |
|
3 |
31 | 15000 | 4.0 | 2.20 | - | + | + | + | NE |
| 4 | 46 | 12000 | 6.0 | 2.90 | + | + | + | + | NE |
| 5 | 27 | 10000 | 3.0 | 2.00 | - | + | + | + | NE |
| 6 | 35 | 5000 | 4.0 | 2.41 | - | + | + | + | NE |
| 7 | 59 | 16000 | 7.0 | 4.55 | + | + | + | + | NE |
| 8 | 20 | 10000 | 5.0 | 2.65 | + | + | + | + | NE |
| 9 | 34 | 50000 | 3.0 | 2.00 | + | - | + | + | NE |
| 10 | 28 | 17000 | 6.0 | 2.70 | + | + | + | + | NE |
| 11 | 27 | 40000 | 4.0 | 2.75 | + | + | + | + | NE |
| 12 | 26 | 15000 | 3.0 | 2.25 | - | + | + | + | NE |
| 13 | 32 | 20000 | 5.0 | 2.80 | + | - | + | + | NE |
| 14 | 35 | 20000 | 8.0 | 4.50 | + | + | + | + | NE +C |
| 15 | 26 | 12000 | 6.0 | 3.05 | - | + | + | + | NE +C |
| 16 | 37 | 30000 | 2.0 | 1.70 | - | - | - | + | NE +C |
| 17 | 43 | 12000 | 4.0 | 2.10 | - | - | - | + | NE +C |
| 18 | 29 | 6500 | 5.0 | 2.40 | - | - | - | + | NE +C |
| 19 | 39 | 25000 | 5.0 | 2.50 | - | + | + | + | NE +C |
| 20 | 25 | 11000 | 4.0 | 2.00 | - | - | - | + | NE +C |
| 21 | 61 | 30000 | 6.0 | 2.65 | + | + | + | + | NE +C |
| 22 | 20 | 7000 | 3.0 | 2.00 | - | - | - | + | NE +C |
|
23 |
31 | 9000 | 8.0 | 3.80 | + | + | + | + | NE +C |
| 24 | 28 | 12000 | 4.0 | 1.74 | - | - | - | + | NE +C |
| 25 | 26 | 10000 | 6.0 | 2.60 | + | + | + | + | NE + W |
| 26 | 34 | 8000 | 7.0 | 4.32 | + | + | + | + | NE + W |
| 27 | 55 | 10,000 | 9.0 | 2.58 | - | + | + | + | NE +ND |
| 28 | 43 | 15,000 | 10.0 | 3.72 | + | - | + | + |
NE + ND |
NE = Necrotic enteritis, NE + C = Necrotic enteritis and Coccidiosis, N + W = Necrotic enteritis and Worm infestation, NE + ND = Necrotic enteritis and Newcastle Disease.
ed to SPSS 17.0 statistical software for analysis. One way ANOVA was used to find out the significant effect of NE on egg production and mortality. Fisher’s exact test was used to find the effect of age, temperature, rainfall, season on the occurrence of NE in commercial layer chicken. The probability value (P) <0.05 was considered as statistically significant.
Results
Among the 100 flocks examined for gastrointestinal lesions, 28 flocks showed NE lesions and intestinal scrapings revealed the presence of large gram- positive rods by Gram’s staining suggestive of C. perfringens. Microscopic examination of intestinal scraping of 11 out of 28 NE affected flocks revealed coccidial oocysts along with Clostridium sp., From oocyst morphology and location, the species was confirmed as Eimeria maxima. However, no gross lesions suggestive of coccidiosis were observed at the time of postmortem. In two flocks, intestine showed the presence of Ascardia galli along with the necrotic enteritis lesions. Lesions suggestive of Newcastle disease and necrotic enteritis was noticed in two flocks and the disease was confirmed by heamagglutination test.
Inoculation of 28 processed intestinal contents in thioglycollate broth produced turbidity and saccharolytic reaction in Robertson cooked meat medium with brain heart infusion broth. Growth on the clostridial agar was obtained on the initial streak from the culture. Selective streaking of these colonies on perfringens agar with supplements revealed rough and black colonies with sulphate reduction, characteristics of C. perfringens organisms.
Grey, flat, round, glistening and double haemolytic colonies on sheep blood agar, classical stormy clot or stormy reaction with excessive gas formation (Figure 1) in milk medium and a zone of opalescence around the colonies on egg yolk agar (Figure 2) were noticed. Smears taken from the colonies when stained by Gram’s method revealed Gram positive, short and plump rods with blunt ends whereas the smears taken from the old culture revealed subterminal, large and oval spores.The isolates were non motile in hanging drop method. All the isolates liquefied gelatin, fermented glucose, maltose, lactose and sucrose except mannitol and negative for oxidase and catalase. Based on the results obtained from the above said tests, the isolates were identified as C. perfringens.
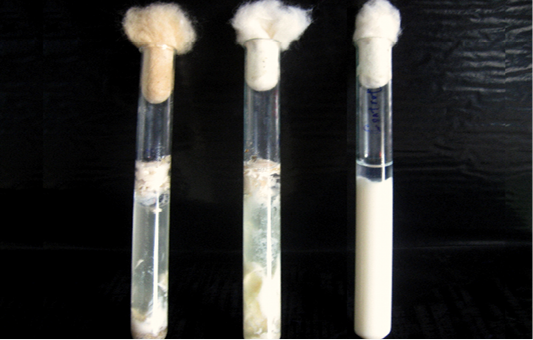
Figure 1: Milk medium showing the classical stormy clot reaction of C. perfringens.The tube on the left extreme is uninoculated.
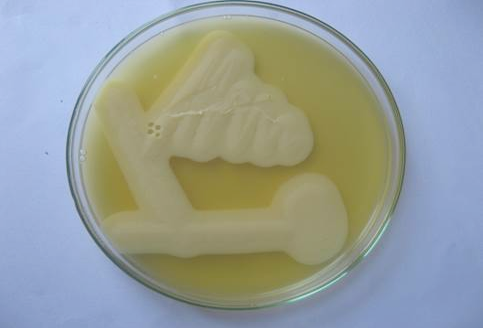
Figure 2: Lecithinase activity of C. perfringens in egg yolk agar after 24 hours of growth. Streaks showing opalescence production around C. perfringens.
All the 28 isolates subjected to alpha toxin specific PCR and produced the predicted amplification size of 775bp (Figure 3), hence all the isolates were proved to be the alpha toxin producing strains of C. perfringens.
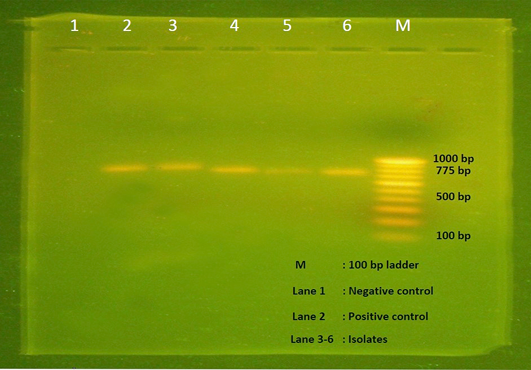
Figure 3: Agarose gel electrophoresis showing 775 bp amplicon of alpha toxin gene of C. perfringens. Lane M- 100 bp DNA ladder, Lane 1- Negative control, Lane 2- Positive control, Lane 3-6 – Isolates positive for C. perfringens.
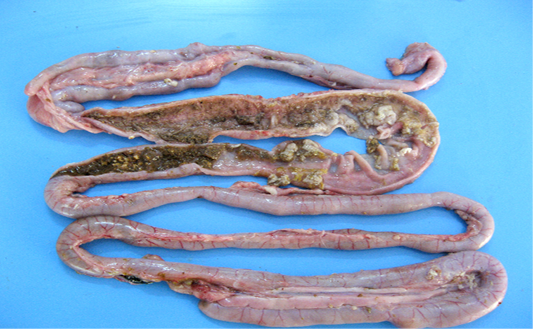
Figure 4: Intestinal mucosa showing diffuse necrosis and pseudomembrane which forming a dirty turkey towel appearance.
Necrotic enteritis affected flocks showed depression, anorexia, reluctance to move, somnolence, cyanotic comb, dehydration and ruffled feathers. Some birds also revealed soiled vent with brownish fecal material adhering to cloaca. Sudden death after showing clinical illness for shorter duration was observed in many flocks. On necropsy examination the intestines were thin walled, friable, dilated and filled with gas. The mesenteric vessels were engorged with blood. On opening, the intestinal lumen contained bile-stained fluid and granular debris. The mucosa was covered with firmly adhering yellowish brown diphtheritic membrane giving a dirty Turkish towel appearance (Figure 4). These lesions were usually confined to the small intestine, primarily jejunum and ileum. Few affected birds also revealed these lesions in the colon, caecum and rectal regions.
Prevalence of necrotic enteritis in commercial layer chicken was presented in Table 1. The disease was detected in 13 flocks (13 %) as single and 15 flocks (15 %) as combined infections with intestinal coccidiosis (11%), Ascaridia galli
Table 2: Age wise prevalence of necrotic enteritis in commercial layer chicken
| S.No. | Age (Wks) | No.of flocks positive | Total | Percentage | ||||
| NE | NE + Cocci | NE + Worm | NE +ND | |||||
| 1 | 21-30 | 6 | 5 | 1 | - | 12 | 42.86 | |
| 2 | 31-40 | 4 | 4 | 1 | - | 9 | 32.14 | |
| 3 | 41-50 | 1 | 1 | - | 2 | 4 | 14.28 | |
| 4 | 51-60 | 2 | - | - | - | 2 | 7.14 | |
| 5 | 61-70 | - | 1 | - | - | 1 | 3.58 | |
| 6 | 71-80 | - | - | - | - | - | - | |
Table 3: Month-wise incidence of Necrotic enteritis in commercial layer chicken for three years
| S.No | Month | Mean Temp (oC) | Mean Rainfall (mm) | No.of flocks positive | Total | |||
| NE | NE + Cocci | NE + Worm | NE +ND | |||||
| 1 | January | 25.3 | 9 | - | 02 | - | - | 02 |
| 2 | February | 27.1 | 10 | - | - | - | - | - |
| 3 | March | 29.4 | 8 | 01 | 01 | - | - | 02 |
| 4 | April | 31.1 | 44 | - | - | - | - | - |
| 5 | May | 31.3 | 88 | - | - | - | - | - |
| 6 | June | 30.2 | 37 | - | - | - | - | - |
| 7 | July | 29.2 | 59 | - | - | - | - | - |
| 8 | August | 28.9 | 97 | - | - | - | - | - |
| 9 | September | 28.7 | 99 | 01 | - | - | - | 01 |
| 10 | October | 27.7 | 184 | 03 | 02 | 01 | 01 | 07 |
| 11 | November | 26.2 | 89 | 05 | 04 | - | - | 09 |
| 12 | December | 25.1 | 40 | 03 | 02 | 01 | 01 |
07 |
infestation (2%) and Newcastle disease (2%). Prevalence of NE in various age groups of layer chicken was presented in Table 2. Incidence of NE was noticed in all age groups of layer chicken, however most commonly noticed in the 21-30 wk layers (42.86%) and it had significant (P > 0.05) effect on the occurrence of the disease. The drop in egg production of 3 to 7, 2 to 8 and 9.5 to 10 % was noticed in NE, NE and parasitic infection and NE and ND affected flocks respectively. NE and ND affected flocks showed significant (P > 0.05) effect on egg production than NE and NE and parasitized flocks. Average mortality of 2.72 %, 2.72 %, and 3.15 %, was observed in NE, NE and parasitic infection and NE and ND affected flocks respectively. Month wise incidence of necrotic enteritis in commercial layer chicken was presented in Table 3. Prevalence of NE was correlated with mean temperature and rainfall. Low temperature had significant (P > 0.05) effect on the occurrence of NE in commercial layer flocks. Most of the NE outbreaks were recorded in the northeast monsoon (17 flocks; 60.71 %) followed by winter (9 flocks; 32.14 %) and summer (2 flock: 7.15%) season. Season wise analysis revealed northeast monsoon had significantly (P > 0.01) higher incidence of NE. Fish meal and meat and bone meal were used as a source of protein in the affected flocks. Cultural examination of fish meal (15/18), meat and bone meal (19/28) and feed samples (22/28) from the necrotic enteritis affected flocks revealed the presence C. perfringens.
Discussion
Enteric diseases are one of the most important problems in the poultry industry because of high economic losses due to decreased production, increased mortality, greater medication costs and increased risk of contamination of poultry products for human consumption (Timbermont et al., 2011). The most common and severe type of enteric disorders in poultry is necrotic enteritis (NE), caused by C. perfringens type A, although in rare cases type C may be involved (Cooper et al., 2013). The disease primarily affects 2 to 6 wk age broilers, while some authors recorded in 3 to 6 months layer chicken. However reports on the occurrence of necrotic enteritis in commercial layer chicken under field condition was meager (Sawale et al., 2010; Malmurugan et al., 2012).
In the present investigation, 28 out of 100 flocks with gastrointestinal disorders showed the lesions of necrotic enteritis and the intestinal scrapings revealed the presence of large gram-positive rods by Gram’s staining suggestive of C. perfringens and it was confirmed by morphological and biochemical characters which was in agreement with earlier work on the isolation (Craven et al., 2001; Malmarugan et al., 2012). C. perfringens is considered as commensal organism of normal chicken intestinal flora (Petit et al., 1997) for this reason, we should differentiate between toxigenic and non-toxigenic isolates. Our results revealed that, all the 28 isolates were alpha toxin producing strains of C. perfringens by PCR technique. Schoepe et al. (2001) also used PCR for diagnosis and differentiation of non toxic and alpha toxin producing strains of C. perfringens by amplifying 775 bp. Alpha toxin has been indicated as a main virulence mediator for NE in poultry and it hydrolyses phospholipids and promotes cellular membrane disorganization leads to destruction of intestinal mucosa (Cooper et al., 2013).
Depression, anorexia, brownish diarrhoea dehydration, cyanotic comb and ruffled feathers revealed in the present study are in agreement with the observations of many authors (Al-Sheikhly and Truscott, 1977; Al-Sheikhly and Al-Saieg, 1980). Sudden death after showing clinical illness for shorter duration was observed in many flocks. This might be due to the highly potent alpha toxin produced by C. perfringens (Naylor et al., 1998; Rood, 1998; Titball et al., 2000). In the present study, gross lesions of friable, thickened mucosa, with firmly adhering yellowish brown diphtheritic membrane in the jejunum and ileum are well supported by the earlier observations of Cooper et al. (2013); Malmurugan et al. (2013). These lesions in the small intestine might be due to the action of toxin produced by C. perfringens (Rood, 1998). Al-Sheikhly and Truscott, (1977) and Kaldhusdal and Skjerve (1996) also observed NE lesions frequently in the jejunum and ileum region of small intestine.
Based on the bacteriological and pathological observation the prevalence of necrotic enteritis in commercial layer flocks were estimated as 28 %. These results are in agreement with Kaldhusdal and Skjerve (1996); Dar et al. (2017), they also reported the prevalence of 27.1 % and 34,8% respectively. Necrotic enteritis was recorded as single (13.0 %) and combined (15 0 %) infections and 11 of the 28 NE positive flocks had concurrent infection of coccidiosis. Intestinal damage during Eimeria infection will result in leakage of plasma proteins (Van Immerseel et al., 2004) and mucin (Collier et al., 2008) into the lumen of the intestinal tract, which is a rich nutrient substrate and favorable medium for C. perfringens proliferation and toxin production.
In the present study NE was commonly noticed between 21 to 30 wk of age. In commercial layers, peak egg production occurs between 21 to 30th wk of age, during this period the birds are subjected to high physiological and hormonal stresses (Srinivasan et al., 2012) due to alteration in feeding regime (moving from a pre layer to layer feed with high calcium content), increases in stocking density, initiation and peak egg production causes GIT stress leads to changes in the intestinal environment in such a way that the risk of induction of NE is increased (McDevitt et al., 2006; Tsiouris et al., 2015).
The drop in egg production of 3 to 7 , 2 to 8 and 9.5 to 10 % in NE, NE and parasitic infection and NE and ND respectively found in the current study were also observed by other researchers (Malmurugan et al., 2012; Cooper et al., 2013; Balachandran et al., 2014). In the present study average mortality of 2.72, 2.72 , and 3.15 %, were noticed in NE, NE and parasitic infection and NE and ND respectively were in agreement with the Porter, (1998); Dhillon et al. (2004); Balachandran et al. (2014). Maximum number of necrotic enteritis cases were recorded in the northwest monsoon (60.71 %) followed by winter (32.14%) and summer (7.15%) season. These findings are in agreement with Craven et al. (2001); Williams (2005). In Namakkal region mean rain fall is high during the northwest monsoon season which increases the relative humidity and moisture content in poultry environment, moreover ambient temperature is also congenial during this season for the survival of C. perfringens and coccidial oocyst.
Upon investigation, it was revealed that fishmeal and meat and bone meal were used in the feed as a source of protein. Cultural examination of fish meal (15/18), meat and bone meal (19/28) feed samples (22/28) from the necrotic enteritis affected flocks revealed the presence C. perfringens. Timbermont et al. (2011) hypothesized that animal protein sources would have higher levels of indigestible protein that would reach the ceca and serve as substrate for C. perfringens. Hence, the source of infection in the present study may be contaminated feed and coccidiosis and stress could have acted as a predisposing factor in some flocks because few feed samples (6/28) were negative for the C. perfringens.
Conclusion
Among the gastrointestinal disorders of commercial layer chicken necrotic enteritis contributed 28 % and it was recorded as single (13 %) and combined 15 (15%) infection with coccidiosis (11%), worm infestation (2%) and Newcastle disease (2%). All the 28 C. perfringens isolates were identified as alpha toxin producing strains by PCR technique. The disorder predominantly noticed in young layers (21 to 30 wk) and northwest monsoon season. Drop in egg production and mortality in the NE affected flocks of current study were 3.0 to 10% and 2.72 to 3.15% respectively. Necropsy revealed dilated intestine with inflammatory exudates and mucosal necrosis. C. perfringens contaminated feed may be the source of infection (22/28) and coccidiosis and stress (6/28) acted as a predisposing factors for NE occurrence. This study provides valuable information on the prevalence and predisposing factors associated with NE occurrence under natural conditions in commercial layer chicken and will be useful for the researchers and veterinarians to develop strategies for both treatment and control of this problem in layer industry and the subsequent human illness resulting from consumption of contaminated poultry products.
ACKNOWLEDGEMENTS
The financial support and facilities provided by the Tamil Nadu Veterinary and Animal Sciences University, Chennai, India are duly acknowledged.
CONFLICT OF INTEREST
The author declare that there is no conflict of interest.
Authors Contribution
The present article is part of PB’s Ph.D., research work. PB conceived and implemented the work. GAB was the major research supervisor. PS and SS were the minor research supervisors who made critical suggestions in carry out the study. All authors participated in draft of the manuscript. TRG critically reviewed the manuscript. All authors read and approved the final manuscript.
References






