Advances in Animal and Veterinary Sciences
Short Communication
Advances in Animal and Veterinary Sciences 2 (5): 292 – 295Carriage of Multiple drug Resistant Bacteria in Vagina of Apparently Healthy Swamp Buffaloes in Nagaland
Bhoj Raj Singh1*, Raj Karan Singh2, Vidya Singh3
- Division of Epidemiology, Indian Veterinary Research Institute, Izatnagar–243 122, India
- Krishi Vigyan Kendra, Porba, National Research Centre on Mithun, Jharnapani–797 106, India
- National research Centre on Mithun, Jharnapani–797 106, India
*Corresponding author: [email protected]
ARTICLE CITATION:
Singh BR, Singh RK, Singh V (2014). Carriage of multiple drug resistant bacteria in vagina of apparently healthy swamp buffaloes in Nagaland. Adv. Anim. Vet. Sci. 2 (5): 292 – 295.
Received: 2014–05–19, Revised: 2014–06–02, Accepted: 2014–06–03
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.5.292.295
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
Isolation of multiple drug resistant (MDR) bacteria with potential of extended spectrum β–lactamases (ESBL) and carbapenemase production from vaginal swabs of apparently healthy swamp buffaloes is reported for the first time. A total of 57 bacterial isolates belonging to 18 species were isolated from vaginal swabs of apparently healthy swamp buffaloes. Escherichia coli was the most common bacteria isolated from 68% samples followed by Enterobacter agglomerans (32%), Klebsiella pneumoniae (16%), Erwinia amylovora (16%), Aeromonas media (12%), Alkaligenes denitrificans (12%) and Aeromonas hydrophila (8%). A total of 35.1 % isolates had MDR and 14 isolates were phenotypically confirmed as producer of crabapenemase or ESBL. Resistance to ampicillin was the most common (68.4%) followed by resistance to ceftazidime (33.3%), nitrofurantoin (31.6%) and cotrimoxazole (21.1%). Resistance to imipenem, aztreonam and ceftazidime + clavulanic acid was detected in 5, 5 and 9 isolates, respectively. The study conclude that vagina of swamp buffaloes may carry several microbes having multiple drug resistance without any apparent illness.
Bubalus bubalus or sometimes named Bubalus arnee fulvus (swamp buffalo) often found roaming under semi–domesticated mode in villages of Nagaland and neighbouring areas of North–East India, Myanmar and Thailand (Groves, 1996). Though several studies have been reported from other parts of the world on health and production aspects of swamp buffaloes (Verin, 2011; Hill et al., 1993; Tongyai, 1993; Tantivanich et al., 1988) information is scant on diseases of Indian swamp buffalo (Singh et al., 2012). Several studies on bacterial flora of reproductive tracts of different animals and humans have revealed role of commensal microbes in innate immune response and health of reproductive tract (Bara et al., 1993; Mshelia et al., 2001; Nelson et al., 2008; Strömbeck, 2008; Zhi et al., 2008; Singh, 2009a; Chotimankul and Sirivaidyapong, 2010). Reproduction failure (infertility) and reproductive tract infections are often the outcome of change in commensal flora of vagina (Larsen and Galask, 1982; Larsen and Gilles, 2001; Singh, 2009a; Chotimankul and Sirivaidyapong, 2010). Besides, bacterial microbiota of vaginal tract may also have profound effect on health of newborns (Srinivasan and Fredricks, 2008). Thus the knowledge of commensal bacteria in vagina of healthy subjects is necessary to give a therapeutically pertinent diagnosis when vaginal cultures are recommended by the veterinarians. This study was undertaken to determine aerobic bacteria in vagina of swamp buffaloes.
Vaginal swab samples (VS) were collected from 25 apparently healthy non–pregnant (diestrus) swamp buffaloes without any history of illness or antimicrobial chemotherapy in Medziphema sub–district area of Dimapur district of Nagaland state. Buffaloes were restrained in cattle–crush and peri–vaginal area was swabbed with a potent non–irritant antiseptic (Triclogel, HiMedia, Mumbai). Sterile swab sticks (Hi–Media) were inserted deep (15 cm) into the vagina and gently rolled around the vaginal walls and withdrawn carefully. Swabs were transferred into Amies charcoal medium (Hi–Media) and brought to the laboratory within 72 h of collection. In laboratory, each swab was aseptically transferred to a tube containing sterile 10 ml buffered peptone water (BBL BD, USA) and incubated at 37oC for 6–8 h. Thereafter, broth culture was inoculated onto 5% sheep blood agar (BA, BBL BD) and Hektoen enteric agar (BBL BD), the plates were incubated at 37ºC for 24–48 h and then observed for growth. Three to five of the well isolated colonies of each morphologically distinct group were picked onto BA plates for further purification. Isolated pure colonies were identified on the basis of Gram staining, morphological, growth and biochemical characteristics (Holt et al., 1994) using standard methods (Singh, 2009b; Carter and Cole, 1990).
Antibiotic sensitivity of all the isolates was determined using disc diffusion methods as per CLSI guidelines (CLSI, 2007) on Muller Hinton agar (BBL BD), using ampicillin 10 µg, tetracycline 30 µg, gentamicin 30 µg, nitrofurantoin 300 µg, cotrimoxazole 25µg, ciprofloxacin 10 µg, chloramphenicol 25 µg, ceftazidime 30 µg,
ceftazidime clavulanic acid 30+10 µg, imipenem 10 µg, azithromycin 15 µg and aztreonam 30 µg discs (BBL BD). Diameter of zone of growth inhibition around antimicrobial disc was measured in mm and isolates were classified as sensitive or resistant according to CLSI (2007) standards. Multiple drug resistance (MDR) of a bacterial strain was defined as resistance to three or more of the antimicrobials tested. All the tests were done in triplicate and a reference E. coli K12 strain (E–382), sensitive to all antibiotic was used as control. From any one animal all the strains having similar morphological, cultural and biochemical characteristics and similar antimicrobial sensitivity pattern were counted as one isolate. For concluding about MDR and for predicting production of antimicrobial drug inactivating enzymes viz., extended spectrum β–lactamases (ESBL) EUCAST guidelines (2013) were followed.
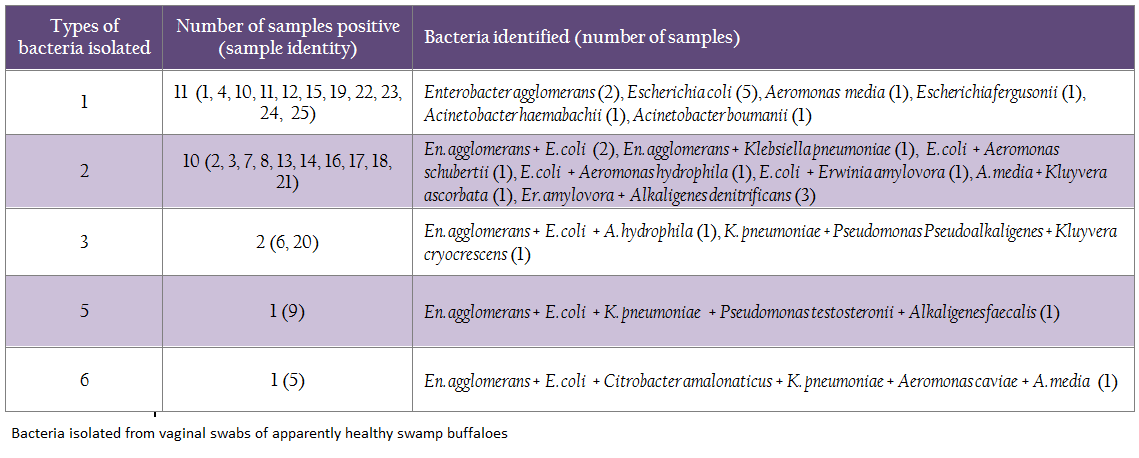
In the study vaginal swabs of all 25 swamp buffaloes sampled were positive for one (11) or more (14) types of bacteria (Table 1). A total of 57 isolates belonging to 18 different species of bacteria were identified in vaginal swabs of apparently healthy swamp buffaloes. Seventeen isolates of Escherichia coli were the most common bacteria isolated from 13 (68%) animals followed by Enterobacter agglomerans (32%), Klebsiella pneumoniae (16%), Erwinia amylovora (16%), Aeromonas media (12%), Alkaligenes denitrificans (12%) and Aeromonas hydrophila (8%). Other bacteria (of 11 species) were isolated from only one samples each (Table 2).
In our study E. coli was identified as the most common bacteria isolated from vagina of swamp buffaloes similar to earlier observations on healthy cows (Zhi et al., 2008), sows (Bara et al., 1993), mares (Singh, 2009a, c) and bitches (Chotimanukul and Sirivaidyapong, 2010). Similar observations have been made on Mithuns in the same regions (unpublished data), and E. coli could be detected in about 43% vaginal swabs of Mithuns. In Swamp buffalo vaginal swabs enterococci were not detected while in Mithuns, enterococci (40%) were second to E. coli in prevalence and Enterobacter were detected only in 10% samples in contrast to 32% of swamp buffalo vaginal swabs. This variation might be due to difference in micro–habitat of or physiology of the vagina of both the animals. The observations of common occurrence of Enterobacter and Klebsiella in vaginal swabs of swamp buffaloes are in concurrence to earlier observations on other healthy animals (Bara et al., 1993; Zhi et al., 2008; Singh 2009 a, c). However, detection of pseudomonads and aeromonads has rarely been reported in vagina of healthy animals except in Mithuns of Nagaland. Similarities in prevalence of aeromonads and pseudomonads in vagina of healthy Mithun and swamp buffalo might be attributed to the same niche and some interaction of both types of the animals; however, needs further studies to confirm. Although En. agglomerans was isolated from healthy buffaloes in the study, it may also be an opportunistic pathogen and has been reported earlier in association with conjunctivitis in swamp buffaloes (Singh et al., 2012).
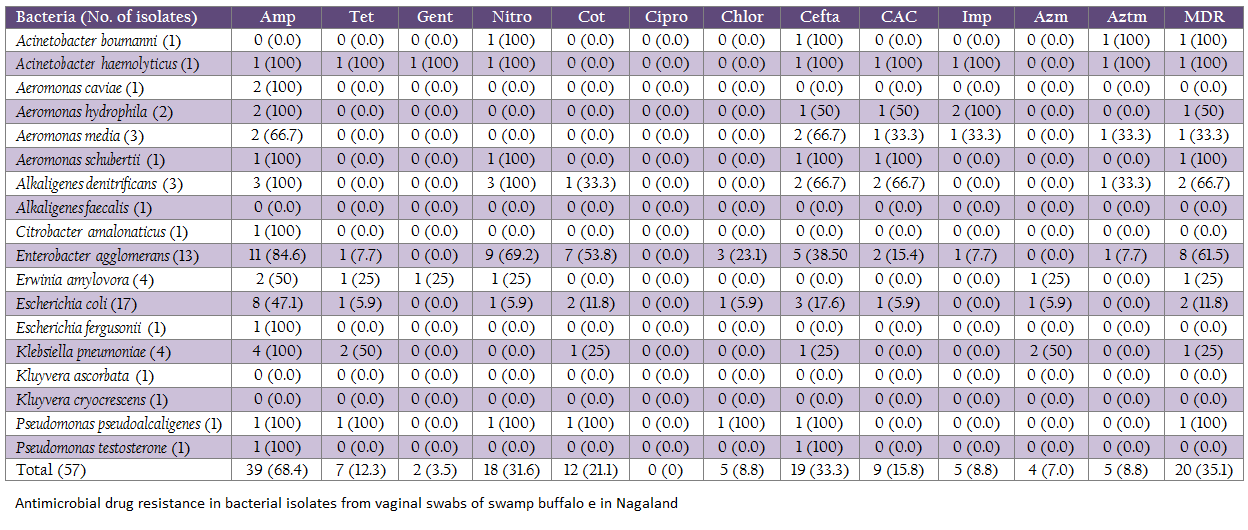
Antimicrobial drug resistance was common in bacteria isolated from vaginal swabs (Table. 2). Although 20 (35.1%) isolates had multiple drug resistance (resistance to three or more antimicrobials), none of 57 isolates had resistance to chloramphenicol. Although E. coli were the most commonly isolated bacteria, only two of 17 strains had MDR. On the other hand, MDR was the most common trait of En. agglomerans (66.7%). The eight isolates of En. agglomerans having MDR were isolated from six different animals and 2, 3 and 3 isolates had resistance to 3, 4 and 5 antimicrobials, respectively. A total of 10 isolates were sensitive to all the antimicrobials used in the study (Kluyvera 2, Erwinia 2, E. coli 5, Alk. faecalis 1) and 16 isolates were resistant to only one antimicrobial while 11 isolates for two antimicrobials. Among the most resistant isolates Acinetobacter haemolyticum was sensitive to only 4 drugs out of the twelve tested, while Pseudomonas pseudoalcaligenes was resistant to 6 drugs. Multiple drug resistance in Acinetobacter, pseudomonads and Alkaligenes strains is often reported but most of such isolates were of nosocomial origin (Singh and Singh, 2012) but rarely from healthy subjects or animals as observed in the present study. The study indicated that MDR strains are entering into remote lands and healthy animals which might be a big problem in future.
Resistance to ampicillin was the most common (68.4%) followed by resistance to ceftazidime (33.3%), nitrofurantoin (31.6%) and cotrimoxazole (21.1%). For other antimicrobials resistance was detected in few isolates. Resistance to imipenem, aztreonam and ceftazidime clavulanic acid detected in 5, 5 and 9 strains, respectively is of public health as well as animal health importance. On the basis of antimicrobial drug resistance pattern (EUCAST, 2013), a total of 5 strains (A. hydrophila, A. media, En. agglomerans, Ac. haemolyticum) were predicted to be producer of carbapenemase, 6 as producer of Amp–C β–lactamase (En. agglomerans, A. hydrophila, A. media, A. schubertii, Alk. denitrificans, E. coli) and four as producer of extended spectrum β–lactamase (ESBL, A. media, Ac. boumanni, Alk. denitrificans, En. agglomerans). Detection of ESBL, Amp–C –lactamase and carbapnemase producing strains in swamp buffaloes is of public health concern because this type of resistance is usually transferable and severely affect the outcome of the treatment (CLSI, 2007; EUCAST, 2013).
Detection of MDR in 35.1% bacteria isolated from vaginal swabs of swamp buffaloes is surprising as antimicrobials might have rarely been used in swamp buffaloes as these animals are seldom treated in Nagaland. However, detection of antimicrobial drug resistant or MDR strains from swamp buffaloes are in concurrence to earlier observation on bacterial isolates from vaginal swabs of mithuns, food, water and other environmental sources in Nagaland (Singh et al., 2012; 2013, 2014a, b) indicating circulation of drug resistant and MDR strains in areas where antimicrobials are not in common use. Many bacteria have been identified in association of vaginal tract through conventional techniques for bacterial culture (DiGiulio et al., 2008; Goldenberg et al., 2008; Nelson et al., 2008; Singh, 2009a) use of molecular methods (Lamont et al., 2011) may further enhance our understanding about role of vaginal micro–biome in health and reproduction. The study conclude that vagina of swamp buffaloes may carry several potentially pathogenic microbes either living as commensal or in pre–infection/ subclinical infection stage having multiple drug resistance without any clinical sign of illness.
ACKNOWLEDGEMENTS
Authors (BRS and RKS) are thankful to Director of NRC Mithun to permit for conducting the sampling and also to the Director Indian Veterinary Research Institute for providing facility to analyze the samples. Technical assistant from Mr. HC Joshi and laboratory assistance from Mr. Laikurahman also needs to be acknowledged.
REFERENCES
Bara MR, McGowan MR, O'Boyle D, Cameron RD (1993). A study of the microbial flora of the anterior vagina of normal sows during different stages of the reproductive cycle. Australian Vet. J. 70: 256–259.
http://dx.doi.org/10.1111/j.1751-0813.1993.tb08043.x
PMid:8368967
Carter GR, Cole JR (1990). Diagnostic Procedures in Veterinary Microbiology, 5nd edn, San Diego, Academic Press Inc. HBC publishers. 1–620.
Chotimanukul S, Sirivaidyapong S (2010). The relationship of canine vaginal and uterine bacterial species in closed–cervix and opened–cervix pyometra. Proceedings of 13th Association of Institutions for Tropical Veterinary Medicine (AITVM) Conference 23–26 August 2010 Bangkok, Thailand, 184–186.
CLSI (2007). Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement. CLSI document M100–S17. Wayne, Clinical and Laboratory Standards Institute. 1–182.
DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA (2008). Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture–based investigation. PLoS ONE. 3: e3056.
http://dx.doi.org/10.1371/journal.pone.0003056
PMid:18725970 PMCid:PMC2516597
EUCAST (2013). EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. European Committe on Antimicrobial Susceptibility Testing. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf
Goldenberg, RL, Culhane JF, Iams JD, Romero R (2008). Epidemiology and causes of preterm birth. Lancet. 371: 75–84.
http://dx.doi.org/10.1016/S0140-6736(08)60074-4
Groves CP (1996). The taxonomy of the Asian wild buffalo from the Asian mainland. Int. J. Mammal. Biol. 61: 327–338.
Hill FI, Arthur DG, Thompson J (1993). Malignant catarrhal fever in a swamp buffalo (Bubalus bubalis) calf in New Zealand. New Zealand Vet. J. 41: 35–38.
http://dx.doi.org/10.1080/00480169.1993.35732
PMid:16031692
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994). Bergey's Manual of Determinative Bacteriology, 9th edn., Baltimore, Williams and Wilkins. 1–1632.
PMid:7510616
Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, Romero R (2011). The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG: An Int. J. Obst. Gynaecol. 118, doi: 10.1111/j.1471–0528.2010.02840.x.
http://dx.doi.org/10.1111/j.1471-0528.2010.02840.x
Larsen B, Galask R (1982). Vaginal microbial flora: composition and influence of host physiology. Ann. Internal Med. 96: 926–930.
http://dx.doi.org/10.7326/0003-4819-96-6-926
PMid:7046547
Larsen B, Gilles MRG (2001). Understanding the bacterial flora of the female genital tract. Clin. Infect. Dis. 32: 69–77.
http://dx.doi.org/10.1086/318710
PMid:11181139
Mshelia GD, Amin, Chaudhary SUR (2001). Vaginal bacterial flora of nigeria local bitches. Agric. Biol. 3: 183–185.
Nelson DB, Bellamy S, Clothier BA, Macones GA, Nachamkin I, Ruffin A, Allen–Taylor L, Friedenberg FK (2008). Characteristicsand pregnancy outcomes of pregnant women asymptomatic for bacterial vaginosis. Maternal Child Hlth. J. 12: 216–22.
Singh BR (2009a). Occurrence of multiple drug resistant (MDR) aerobic bacteria in vaginal swabs of mares and their association with infertility. Indian J. Comp. Microbiol, Immunol. Infect. Dis. 30: 105–112.
Singh BR (2009b). Labtop for microbiology laboratory. Berlin, Lambert Academic Publishing, Ag and Co. 1–83.
Singh BR, (2009c). Thermotolerance and multidrug resistance in bacteria isolated from equids and their environment. Vet. Record. 164:746–750.
http://dx.doi.org/10.1136/vr.164.24.746
PMid:19525523
Singh BR, Singh B (2012). Carriage of carbapenemase resistant mdr strains of acinetobacter baumannii and Staphylococcus carnosus on Hands of Surgeons in Bareilly, India, Noto–are Med. (17128122). 2012–07–19. http://www.notoare.com/17128122.
Singh BR, Singh V, Ebibeni N, Singh RK (2013). Antimicrobial and herbal drug resistance in enteric bacteria isolated from faecal droppings of common house lizard/gecko (Hemidactylus frenatus). Int. J. Microbiol. doi:10.1155/2013/340848, 1–8.
http://dx.doi.org/10.1155/2013/340848
Singh BR, Singh V, Ebibeni N, Singh RK (2014a). Maternal Transfer of Bacteria to Eggs of common house gecko (Hemidactylus frenatus). J. Microbiol. Res. 4: 78–85.
Singh BR, Singh V, Singh RK (2012). Multiple drug resistant Enterobacter agglomerans and Pseudomonas aeruginosa causing conjunctivitis in swamp buffaloes in Nagaland. Noto–are Med. https://www.notoare.com/index.php/index/explorer/getPDF/14612376.
Singh BR, Singh V, Singh RK, (2014b). Microbial quality and safety of axone (akhuni), a fermented soybean food of Nagaland. Noto–are Med. 15185525: 2014–03–14. http://www.notoare.com/15185525.
Srinivasan S, Fredricks DN (2008). The human vaginal bacterial biota and bacterial vaginosis. Interdisciplinary Perspec. Infect. Dis. doi:10.1155/2008/750479. Pp, 22.
http://dx.doi.org/10.1155/2008/750479
Strömbeck L (2008). Vaginal commensal bacteria: Interactions with cervix epithelial and monocytic cells and influence on cytokines and secretory leukocyte protease inhibitor, SLPI. Ph.D thesis, Sweden, Gothenburg University. 1–52.
Tantivanich P, Suttipong P, Naweparp O (1988). Conjunctival flora of normal Thai swamp buffalo. Thailand J. Vet.Med. 18: 159–165.
Tongyai S (1993). Food losses due to Nutritional and metabolic diseases and programme for their reduction. In: Food Losses Due to Non–infectious and Production Diseases in Developing Countries: Proceedings of an FAO Expert Consultation, Rome, 24–28 June 1991. 114: 135–143.
Verin BC (2011). The possible role and significance of carrier swamp buffalo in the transmission of Foot and Mouth Disease in South East Asia (SEA). Ph.D. Thesis, Murdoch University, Dubai. http://researchrepository.murdoch.edu.au/10898/2/02Whole.pdf. 1–176.
Zhi LB, Jun ZX, Yu MZ, Qing ZK (2008). Difference of aerobic and facultative anaerobic bacteria in vagina of postpartum healthy cows and endometritis cows. J. Northwest Agric. Forest. Uni. 11: 57–60.