Advances in Animal and Veterinary Sciences
Research Article
Branchial Cyst in Buffalo (Bubalus Bubalis): Diagnosis and Surgical Treatment
Alaa M.k. Moustafa1, Mohamed A. Hamed2, Hazem Hamouda3, Walid Abdo4, Sabry A. EL-Khodery5*
1Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Kafr- Elshiekh University, Kafr-Elshiekh, 33516, Egypt; 2Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Aswan University, Egypt; 3Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Aswan University, Egypt; 4Department of Pathology, Faculty of Veterinary Medicine, Kafr-Elshiekh University, Kafr-Elshiekh, Egypt; 5Department of Internal Medicine, Infectious and Fish Diseases, Faculty of Veterinary Medicine, Mansoura University, Mansoura 35516, Egypt.
Abstract | The current study aimed to investigate the most common surgical conditions in the pharyngeal area of buffalos through their diagnosis and further surgical management. Ten buffalos were attempted with smooth, fluctuating, painless swelling at the upper part of the neck. Exploratory cyst puncture showed the presence of milky fluid, bloody fluid and yellow viscous fluid in 6, 2 and 2 cases, respectively. Ultrasonography and histopathological examinations were performed to confirm the diagnosis of branchial cysts. Surgical excision was a controversial procedure. Branchial cyst ultrasonography showed an anechoic, fluid-filled structure with a hyperechoic capsule. Numerous tiny hyperechoic fimbriae were found to travel easily inside the liquid when the mass was palpated instantaneously. These were interpreted as potential fibrin or septa tags. Histologically, the cyst was lined with a simple squamous keratinized epithelium that greatly imitates the tissues of the skin. The hairy structure showed keratinized papillary tissues. The cyst under the epithelial layer was lined with mucous glands mixed with a few serous forms. The connective tissue was abundant in both blood vessels and nerve fibers. Surgical intervention to the handle the branchial cyst was curative without recurrence in any of these animals. Additional, exploratory puncture and ultrasonography provide accurate diagnosis and preoperative preparation for branchial cyst care in buffalo.
Keywords | Branchial Cyst, Histopathology, Ultrasonography, Buffalo
Received | October 14, 2020; Accepted | December 12, 2020; Published | March 01, 2021
*Correspondence | Sabry A El-Khodery, Department of Internal Medicine, Infectious and Fish Diseases, Faculty of Veterinary Medicine, Mansoura University, Mansoura 35516, Egypt; Email: [email protected]
Citation | Moustafa AMK, Hamed MA, Hamouda H, Abdo W, El-Khodery SA (2021). Branchial cyst in buffalo (bubalus bubalis): diagnosis and surgical treatment. Adv. Anim. Vet. Sci. 9(5): 631-636.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.631.636
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 El-Khodery et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Buffalo (Bubalus bubalis) is a vital component of the livestock of Egypt that makes a major contribution to farming, provides milk, meat and skin and serves as a draught animal. Compared to cows, the importance of buffalo is linked to better longevity, higher dry milk content and rich organic resistance (Abdellatif et al., 2018).
The most common surgical complications, such as superficial buffalo swelling, include abscesses, hematomas, hernias, bursa, urethral diverticula, and neoplasms. In general, effective clinical identification of such swellings is carried out on the basis of their physical position, palpation and needle aspiration. It is often difficult, however, to assess superficial swelling in buffaloes inside the pharyngeal region by certain traditional clinical techniques only because of their location, the enormous size, behavior of the animal., and the pain associating such lesion (Sagar et al., 2010).
Branchial cysts are a fluctuating, painless mass of buffaloes in the distal ventral neck region (Misk et al., 1994). In both adult and juvenile horses, branchial residual cysts are a rare cause of throatlatch region masses. There are at least four theories regarding their origins, but it is generally recognized that these defects originate from the remains of the fetal branchial cleft, arch, or pharyngeal pouch or from a mixture of these prenatal tissues (Koch, 2005; Nolen-Walston et al., 2009). Branchial abnormalities arise from incomplete obliteration or inability to decay of these clefts and pouches (Waldhausen, 2006) and occur as cysts, sinus tracts or fistulae (Maran and Buchanan, 1978). Branchial cysts are rare embryonic abnormalities in horses, mice, cats, dogs and cattle (Smith and Gunson,1977; Clark et al., 1989; Joffe, 1990; Hance et al., 1992; France et al., 2000).
Ultrasonography is an effective technique for providing precise preoperative information on the degree and character of the surface swelling being examined as it can discriminate fluid from solid deposits and provide an accurate guidance for needle aspiration or biopsies. Ultrasonography can identify much of the space that comprises soft tissue masses from the adjoining tissues, based on identifiable interfaces and enhanced echogenicity, whereas other clinical tests are inconclusive (Kumar et al., 2014). Ultrasound examination of the branchial cyst in horses confirmed the presence of an anechoic fluid-filled cavity with minor hyperechoic fluctuating specks and a well-defined hyperechoic capsule (Rinnovati et al., 2018).
There are two approaches to treat branchial cysts as surgical removal or marsupialization, and iodine solution sclerotherapy (Field et al., 1990; Slovis et al., 2001). The standard treatment for branchial system defects is overall surgical excision, since these lesions do not heal spontaneously. The complete surgical excision of the branchial cysts in the horse may be difficult due to the proximity of vital organs such as the recurrent laryngeal nerve, vagosympathetic trunk, jugular vein and carotid arteries. (De Estrada and Schumacher, 2013). In dogs, the first method was unsuccessful in treating the branchial cyst by incision, aspiration and drainage. In comparison, a full mass surgical excision using a carbon dioxide laser resulted in rapid recovery (Roux and Kuehn, 2013).
Though, branchial cysts have been designated in mice (France et al., 2000), cattle (Smith and Gunson, 1977; Hennig and Steckel, 1991), dogs (Clark et al., 1989), foals (Hance et al. 1992; David et al. 2008), as well as horses (Rinnovati et al., 2018). To date the available literatures, contain limited information on branchial cysts in buffalos. The present study was designated to evaluate the outcomes of surgical treatment and the histopathological structure of branchial cysts in buffalos.
MATERIALS AND METHODS
Buffalos
A total of ten adult buffalos (9 females and 1 male), with an age range of 2-7 years, were presented for visible mass. In each buffalo a smooth, fluctuating, painless swelling was observed in the subcutaneous tissue behind the mandibular angle at the upper part of the neck (Figure 1, A). Over these cysts the skin was not tense, swollen, or inflamed. Initially, the swellings in these animals were detected two months before admission time. Exploratory cyst puncture showed the presence of milky fluid, bloody fluid and yellow viscous fluid in 6, 2 and 2 cases, respectively. This inquiry was permitted by the committee of animal welfare and ethics, Kafr-Elshiekh University.
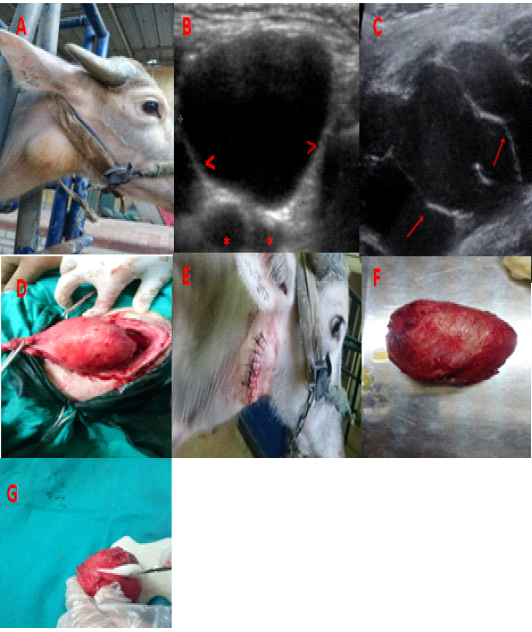
Figure 1:A) Branchial cyst in a 2 years-old buffalo just behind the mandibular angle. B) and C) ultrasonography of brachial cyst wall (>), bifurcation of common carotid artery (*) and septa (arrow). D) blunt dissection of the cyst from the surrounding structure. E) suture of the skin after the end of surgery. F) the cyst after removal. G) milky fluid from the cyst after excision.
Ultrasonographic Examination
A 7.0 – 10.0 MHz mechanical linear and 2.0 – 5.0 MHz curvilinear multi-frequency scanner (Mindray DP-2200Vet., Mindray, Shenzhen, PR China) was used to perform the ultrasonographic examinations, while the animal is in standing position. The seat to be inspected in each of the admitted buffalo was prepared by the clipping and shaving of the hair, followed by an application of coupling gel (Ultragel, Medilab, Cairo, Egypt) above the swelling and neighboring area. The transducer was reallocated dorsoventrally and craniocaudally beginning from the healthy area towards the swelling area. The investigated buffalo was actually sedated using intravenous xylazine HCl at a dose rate of 0.05 mg/kg (Xylaject 2% −ADWIA Co., EL Obour, Egypt).
Surgical Intervention
At a dose rate of 0.05 mg/kg body weight, Buffalo was pre-medicated with xylazine and aseptic surgery was scheduled at the site of the procedure. A longitudinal incision was accompanied down to the sac wall through the skin and underlying tissue. To isolate the sac from the neighboring tissues, blunt dissection was performed. The sac wall was incredibly small, thus it took a lot of time and effort to distinguish it from the important structures around it. The large blood vessels and nerves were released from the cyst wall and held sideways until the procedure was completed (Figure 1, D). The subcutaneous tissues were sutured using coated polyglactin 910 No. 2 (Ethicon; Johnson& Johnson, Brussels, Belgium) in sub epidermal pattern. The extreme skin was trimmed, and the skin was closed with silk No. 2 (Silk, Proadvantage, Co., Cairo, Egypt) in simple interrupted pattern (Figure 1, E).
Postoperative Care and Fellow-Up
Following surgery, each animal received an intramuscular injection of 30,000 IU/kg penicillin G procaine day after day for 10 days postoperatively (Norocillin L.A, Norbrook Lab. Ltd., Newry, Ireland) and 2.2 mg/kg Flunixin meglumine (Flunidin, Arabcomed, El obour Area, Egypt) for 5 days after surgery. The buffalos were restrained in a cubicle rest for two weeks and checked every day for restorative improvement. To assess the long-term results (six months) via a telephone connection with the owners and they were asked about recurrence of cyst, discharge from the surgical location, wound dehiscence and cosmetic appearance.
Histopathological Examination
Next to the surgical procedures, tissue specimens were fixed in 10% neutral buffered formalin, dehydrated in a graded alcohol series, cleared in xylene and then implanted in paraffin wax. Five-micron thick sections were cut and stained with hematoxylin and eosin (Bancroft and Stevans, 1990).
RESULTS
Regarding the recorded clinical signs, the branchial cyst gradually turned up without any obvious signs of inflammation. Soft fluctuating swelling in the upper portion of the buffalo neck was observed (Figure 1, A). Exploratory cyst puncture showed the presence of a milky fluid, bloody fluid and yellow viscous fluid in 6, 2 and 2 cases, respectively. and produced little white flakes which can be easily compressed with finger pressure over the swelling. The clinical symptoms of branchial cysts and seats of predilection in all types were diagnostic, and exploratory puncture was extremely confirmative. The total volume of aspirated fluid from the excised sacs was varied between 70 and 100 ml. Recovery was imprecise in eight cases, and sutures were removed 7 to 10 days post-operative. However, two cases showed a slight residual swelling of the surgical site, but handled good and healthy. Six months post-surgery, all buffalos exhibited no sign of recurrence and appeared to have totally recovered.
Ultrasonography (Figure 1, B and C) was implemented in all 10 clinical cases and the mass displayed anechoic, fluid-filled structure with a hyperechoic capsule. Several tiny hyperechoic fimbriae were found to travel freely inside the fluid when the mass was palpated concurrently. These were defined as potential fibrin or septa marks.
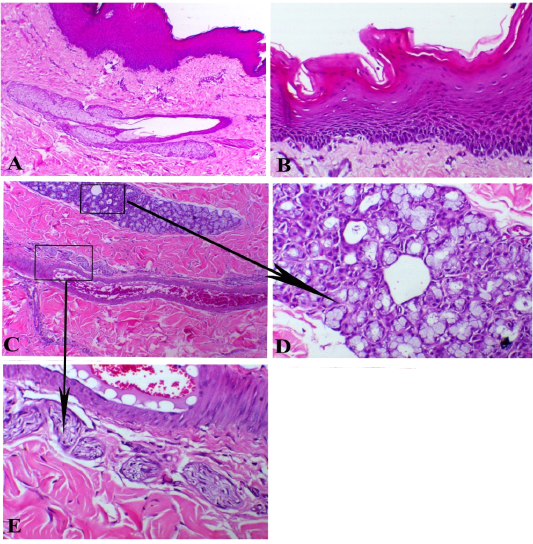
Figure 2: Branchial cyst, A) showed stratified epithelial lining with sub-epithelial sebaceous glands, X100. B) papillary-like stratified squamous epithelium, X200. C) sub-epithelial glandular, vascular and neuronal appendages (arrows indicates insets of both mucous glands and nerve bundles), X 100. D) mucous glands, X200. E) neuronal structure around the blood vessels, X200. H&E stain.
Histologically, the cyst was lined with simple squamous keratinized epithelium that greatly copycatting the skin tissue. The hairy structure revealed papillary keratinized tissues (Figure 2, A and B). Under the epithelial layer, the cyst showed plenty number of mucous glands mixed with scarce serous glandular type (Figure 2, C and D). There connective tissue was rich in both blood vessels and nerve fibers (Figure 2, E). One case showed features of serous cyst that revealed a large cavity filled with serous exudate with scant RBCs separated with delicate fibrous septae. The wall of cyst consisted of fibrous capsule rich in fibrin bands and the outer tissues displayed noticeable edema (Figure 3, A). Also, one case demonstrated hemorrhagic cysts that exhibited intensive hemorrhage inside the cavity. Some of these cysts confirmed organization attempts with marked angiogenesis and fibroblastic cells proliferation at the margin of the cyst (Figure 3, B).
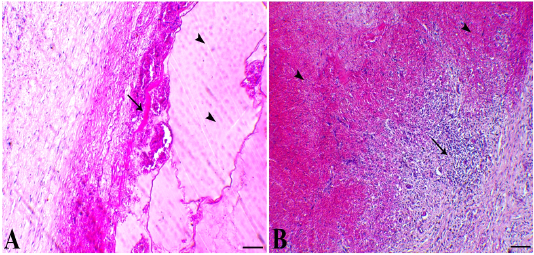
Figure 3: A) cystic structure filled with serous exudates (arrowheads indicates exudate and arrow indicates fibrous capsule of the cyst rich with fibrin bands, X100. B) haemorrhagic cyst showed intensive haemorrhage with organization attempts at the border of the cysts papillary-like stratified squamous epithelium, X100. H&E stain.
DISCUSsION
Branchial cysts are one of the most frequently superficial swellings in buffalo in the cervical zone. Definitive diagnosis of superficial swelling in food producing animals is regularly conducted via case history and clinical investigation. Conversely, it is typically more difficult to diagnose in buffalo than other species of cattle, since they have tougher skin and express less signs of pain (Abouelnasr et al., 2016). This makes the clinical diagnosis more complicated and requires the use of complementary non-invasive medical imaging procedures to validate the provisional diagnosis.
Branchial cysts arise from the remnants of the foetal branching apparatus during embryonic development. In dogs, the branchial duct II, the pharyngobranchial duct II, and the cervical vesicle derived from the branchial clefts III and IV are the structures most susceptible to the implementation of the branchial cysts. In horses, branchial cysts are inferred from remnants of the foetal cleft, arch, and pharyngeal pouch or from a conjunction of these foetal tissues (Slovis et al., 2001). Branchial cysts derived from the ectodermal division cleft are more likely to be coated with stratified squamous epithelium, whereas branchial cysts derived from the entodermal pharyngeal pouch are coated with pseudostratified columnar epithelium (Haruey, 1985; Acierno and Waldhausen, 2007).
Ultrasonography is a somewhat proprietary imaging technique for the soft tissue structure of a buffalo body. It offers a non-invasive, healthy, quick, clear, reliable and dynamic technique for the differential diagnosis of a wide variety of surface swelling types in standing animals, especially when the physical checkups are inaccurate (Ali and Abd El-Hakiem, 2012; Abouelnasr et al., 2016). The diagnostic use of ultrasound in buffalo with superficial swelling has enabled the differential diagnosis and preoperative surgery preparation and ensure correct prognosis.
The differential diagnosis of branchial cysts from other swellings (cervical abscesses, hematomas, tumours, and mucoceles) can be precisely verified by the characteristics of the aspirated fluid as well as by ultrasonography. In the current study, the milky nature of the aspirated fluid from the cysts was highly diagnostic as previously described (Misk et al., 1994). However, the components of branchial cysts in dogs were generally a dense, soft, and sticky liquid. Often the contents were a rust-colored and fearless fluid (Clark et al., 1989). More than ever, ultrasonography of branchial cyst in the examined buffalo showed an anechoic, fluid-filled structure with a hyperechoic capsule as defined in horses (Rinnovati et al., 2018). The echogenicity of abscess components ranged from hypoechoic to anechoic with an echogenic septae, while the contents of hematomas displayed increased echogenicity coupled with the appearance of certain echogenic shreds. However, tumors were identified a round mass with an extremely hyperechoic acoustic shadow. The cervical mucocele proved to be a circular, echogenic structure with a huge quantity of central anechoic material. Moreover, the wall was clearly defined as a hyperechoic structure surrounding the gland (Torad and Hassan, 2013).
In the current study, the surgical total excision of the branchial cyst is the most convenient treatment for buffaloes to prevent its recurrence (Hennig and Steckel, 1991). In previous studies conducted on dogs, the surgical total excision of the branchial cleft cyst appeared to be preferable. However, the infected cyst was noted to drain, first, the pus combined with the antibiotic, and then the excision is a subsequent challenge. Meanwhile, marsupialization or incision and drainage induced unjustifiable healing and often recurrence (Roux and Kuehn, 2013). In horses, surgical removal or marsupialization and sclerotherapy with iodine solution are the most desirable approaches for the treatment of branchial cyst (Hance et al., 1992). Total surgical excision of the branchial cysts may be challenging due to the location of critical structures such as recurrent laryngeal nerves, vagosympathetic trunks, jugular veins and carotid arteries (Slovis et al., 2001). Failure to destroy the cyst lined with the sclerotic agent, 1% of povidoneiodine solution, may lead to relapse, and it may be essential to extract the cyst lining by surgery at the end of therapy (Phelan et al., 1999).
Branchial cysts may be coated either by columnar ciliated (respiratory-type) epithelium or by non-keratinizing stratified squamous epithelium as previously described (Meuten, 2002). In the present study, the branchial cyst was lined with simple squamous keratinized epithelium. In horses, the specimen of the branchial cyst was composed of a vascularized connective tissue wall supplemented with a pseudostratified columnar to cuboid epithelium. Tiny numbers of lymphocytes and plasma cells were found surrounding small mural blood vessels and underlying the epithelium (Slovis et al., 2001).
Branchial cysts should also be distinguished histologically from other cysts at similar locations. Salivary mucocele is an accumulation of saliva that has escaped from the weakened salivary gland or salivary tract and collected in the tissues. Mucocele is coated with granulation tissue, while the cyst is lined with epithelial (glandular) tissue (Roux and Kuehn, 2013). In comparison to the branchial cysts, the epithelium lining the true salivary cysts is neither of the respiratory nor squamous form. Thyroglossal duct cysts coated with pseudostratified ciliated epithelium or squamous epithelium, but are found in the sagittal cervical zone, not on the side of the neck. Parathyroid cysts are lined by multilayered thyrogenic epithelium that regularly has colloid-containing follicles and may be situated close the midline from the base of the tongue caudally into the mediastinum (Meuten, 2002).
The drawback of the current study should be stated. First, the sample size of the examined buffalos was limited, which does not permit a definitive decision. Second, the assessment of the surgical excision of the branchial cyst was viewed on its own. A comparative examination of other surgical procedures for the treatment of branchial cysts is required in order to reach a specific conclusion.
CONCLUSION and CLINICAL PREVALENCE
Surgical excision of the branchial cyst is the most effective method for treating them in buffalo to preclude its recurrence. Additional, exploratory puncture and ultrasonography provide accurate diagnostic skills and preoperative preparatory tools for the care of branchial cyst in buffalo.
acknowledgements
The authors would like to acknowledge the veterinarians in veterinary clinics, buffallos farms who helped in cases collection
conflict of interest
The authors have declared no conflict of interest.
authors contribution
All authors contributed equally to study design methodology, interpretation of results, and writing of the manuscript.
REFeRENCES






