The Journal of Advances in Parasitology
Research Article
Seasonal Occurrence and Infectivity of Nematode Parasites in the Indian Bull Frog, Hoplobatrachus tigerinus Daudin, 1803 (Anura: Dicroglossidae) of YSR (Kadapa), Andhra Pradesh, India
Hemalatha Mannela, Srinivasa C Kalyan, Anuprasanna Vankara*
Department of Zoology, Yogi Vemana University, Vemanapuram, YSR (Kadapa) district Kadapa- 516003, Andhra Pradesh, India.
Abstract | The Indian bull frog, Hoplobatrachus tigerinus Daudin, 1803 from the YSR (Kadapa) District, Andhra Pradesh was found to be infected with the two nematodes, Cosmocercoides variabilis Harwood, 1930 of the family Cosmocercidae and Oswaldocruzia filiformis Goeze, 1782 of the family Molineidae. These two nematodes are the first geographical reports from this region. The seasonal occurrence of these nematode parasites was analysed during the period of February 2013 to February 2014. Of 130 hosts examined, 40 frogs (30.7%) were infected with 305 nematodes i.e. 25 males (n=241) and 15 females (n=64). Intensity of infection ranged from 1-53. C. variabilis (n=199, range= 1-19) was found to be the most common species when compared to O. filiformis (n=106, range=1-48). The effect of locality and seasonal fluctuations on the nematode parasitic loads was observed. Organ wise distribution of these nematodes was analysed along with the influence of host size and sex on the intensity of infection.
Keywords | Hoplobatrachus tigerinus, Oswaldocruzia, Cosmocercoides, Prevalence, Mean intensity, Mean abundance, Index of infection
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | June 17, 2015; Revised | August 01, 2015; Accepted | August 04, 2015; Published | August 10, 2015
*Correspondence | Anuprasanna Vankara, Yogi Vemana University, Vemanapuram, YSR, Andhra Pradesh, India; Email: [email protected]
Citation | Mannela H, Kalyan SC, Vankara A (2015). Seasonal occurrence and infectivity of nematode parasites in the Indian bull frog, Hoplobatrachus tigerinus Daudin, 1803 (Anura: Dicroglossidae) of YSR (Kadapa), Andhra Pradesh, India. J. Adv. Parasitol. 2(2): 34-39.
DOI | http://dx.doi.org/10.14737/journal.jap/2015/2.2.34.39
ISSN | 2311-4096
Copyright © 2015 Mannela et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Anuran amphibians have a rich parasite fauna due to their typical association with both aquatic and terrestrial habitats and are easily susceptible to microbial and parasitic infections. The nematode parasites of amphibians usually have a direct life-cycle without an intermediate host. Survival of infective stages of parasites is inter-related to the moist habitat fondness of amphibians (Prudhoe and Bray, 1982; Jamdar et al., 2010; Nworah and Olorunfemi, 2011). Anuran frogs and toads are abundantly found in the study area due to the warm and humid climates of YSR (Kadapa) District, Andhra Pradesh. Studies on parasites of anurans from this area are totally neglected. Hoplobatrachus tigerinus (Daudin, 1803) of the family Dicroglossidae (Anderson, 1871) is the frequently occurring aquatic species of anurans dwelling in the low lying areas like ponds, ditches and irrigated fields of Kadapa region (Hemalatha et al., 2015). Nematodes of the genus Cosmocercoides wilkie, 1930 and Oswaldocruzia Travassos, 1917 are intestinal parasites of amphibians and reptiles distributed worldwide. Nematode parasites in different species of amphibians were studied by many workers worldwide (Galli et al., 2001; Bursey and Goldberg, 2005, 2006; Anderson, 2000; Jamdar et al., 2010; Nworah and Olorunfemi, 2011; Begum and Banu, 2012; Svitin and Kuzmin, 2012; Gonzalez and Hamann, 2004; Chandra and Gupta, 2007; Mashaii et al., 2008; Jamdar et al., 2012; Guerrar, 2013; Araujo et al. 2014). In the present study, two nematode parasites, Cosmocercoides variabilis Harwood, 1930 of the family Cosmocercidae and Oswaldocruzia filiformis Goeze, 1782 of the family Molineidae were the first geological report from this region frequently infecting H. tigerinus. Very few workers have studied on the seasonal occurrence of these parasites (Plasota, 1969; Baker, 1979; Al-bawari et al., 1980; Wong and Bundy, 1985; Koller and Gaudin, 1997; Jamdar et al., 2010; Chandra and Gupta, 2007). In this study we made an attempt to examine the impact of sex, size, habitat and seasonal changes on the infection by the two species of nematodes in H. tigerinus.
MATERIALS AND METHODS
Sampling Sites
Three different localities where natural vegetation’s are disturbed by anthropogenic activities were selected:
Site A: Industrial Estate area (Lat. 14°47´N 78°76´E, 138 m Altitude), YSR (Kadapa) district
Site B: Ramapuram village (14.05°N 78.75°E, 143 meters), Raychoti and
Site C: Campus area of Yogi Vemana University (Lat. 14°28´N 78°49´E, 137 m Altitude), located in YSR Kadapa District of Andhra Pradesh
All three sites were sampled during February, 2013 to February, 2014.
A total of 130 Hoplobatrachus tigerinus were collected and transported alive to the laboratory. They were anaesthetized with chloroform. Snout-vent length and sex of each frog were recorded. Stomach, intestine and rectal parts were thoroughly examined for the presence of nematodes parasites under the stereozoom microscope (LM-52-3621 Elegant). The collected parasites were stored in 70% ethanol or looss fluid (9 parts 70% ethanol and 1 part glycerol) for further studies. Specific characters were observed under the Lynx trinocular microscope (N-800M) and figures were drawn with the aid of attached drawing tube. All the measurements were taken in millimeters with the help of an ocular micrometer. Pearson’s coefficient of correlation ‘r’ was applied to study the relationship between host’s snout-vent length and parasitic abundance. The influence of host sex on the abundance and prevalence of infection was analysed by employing Chi-square test. Standard statistical books like Snedecor and Cochran (1967), Sundara and Richards (1996) and Daniel (1998) were followed for the quantitative analysis of the data. Various statistical computations like prevalence, mean intensity, mean abundance, standard deviation, correlation, Chi-square test were carried out using Microsoft Excel (2007) and ecological terminologies were adapted from Margolis et al. (1982) and Bush et al. (1997). The impact of habitat preference or locality of host, size, sex and organ wise distribution of parasites on the frequency of parasite populations was analysed.
RESULTS AND DISCUSSION
The seasonal occurrence of the two species of nematode parasites was analysed during the period February 2013 to February 2014. Of the 130 hosts examined, only 40 30.7%) were found to be infected with 305 (range = 1-53) parasites of which 199 (range = 1-19) being Cosmocercoides variabilis and 106 (range = 1-48) being Oswaldocruzia filiformis. The prevalence of infection with C. variabilis is 30 %, showing a mean intensity of 5.10±3.6, mean abundance of 1.53±1.08 and an index of infection (0.45). On the other hand, only 11 frogs (8.46%) showed infection with O. filiformis having a mean intensity of 9.63±6.81, mean abundance of 0.815±0.57 and an index of infection 0.06 (Table 1). 30 frogs (23.07%) were infected with single parasitic species and 10 frogs (7.69%) were infected with both the parasitic species.
Table 1: Infectivity of Nematode parasites in H. tigerinus (n=130)
|
Name of the parasite |
Infected frogs |
Total no. of parasites |
Prevalence (%) |
Mean intensity |
Mean abundance |
Index of infection |
Range |
Location |
|
Cosmocercoides variabilis |
39 |
199 |
30 |
5.10±3.6 |
1.53±1.08 |
0.45 |
1-19 |
Stomach, IntestineRectum |
|
Oswaldocruzia filiformis |
11 |
106 |
8.46 |
9.63±6.81 |
0.815±0.57 |
0.06 |
1-48 |
IntestineRectum |
Table 2: Infectivity of nematode parasites in H. tigerinus from different localities
|
Locality |
No. of hosts examined (a) |
Parasites recovered |
|||||||
|
Cosmocercoides variabilis |
Oswaldocruzia filiformis |
||||||||
|
No. of infected hosts (b) |
No. of parasites (c ) |
Prevalence (%) |
Mean Intensity |
No. of infected hosts |
No. of parasites |
Prevalence (%) |
Mean Intensity |
||
|
Site-A: Industrial Estate, Kadapa |
113 |
35 |
165 |
30.9 |
4.71 |
10 |
58 |
8.84 |
5.8 |
|
Site-B:YVU campus |
12 |
3 |
29 |
25 |
9.66 |
0 |
0 |
0 |
0 |
|
Site-C:Ramapuram |
5 |
1 |
5 |
20 |
5.0 |
1 |
48 |
20 |
48 |
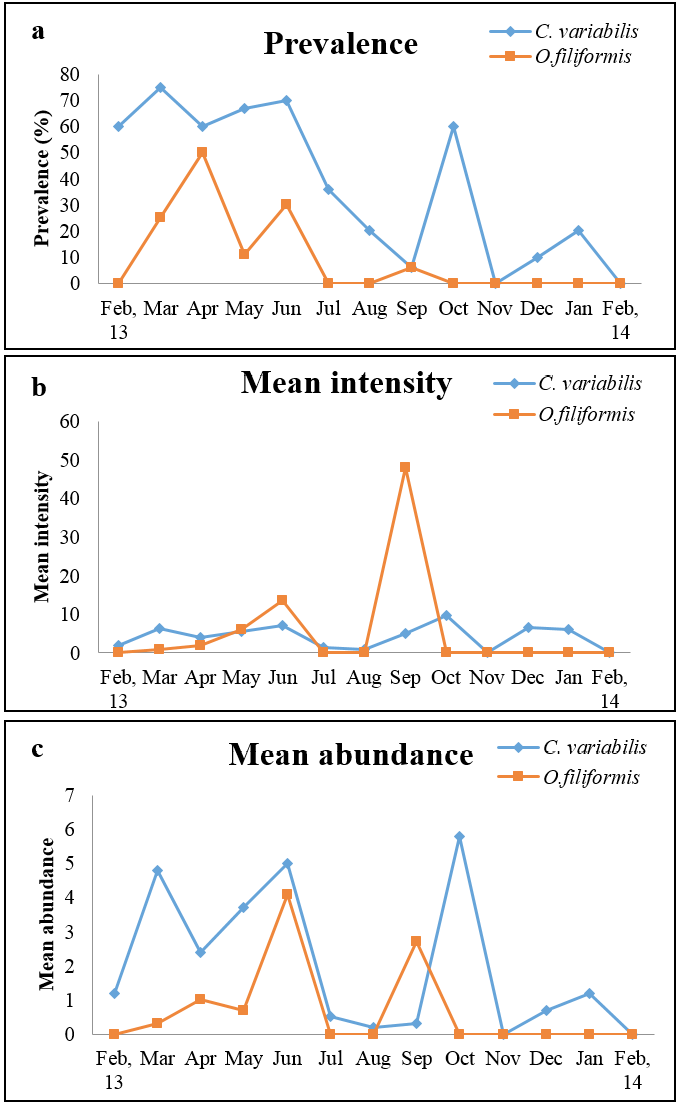
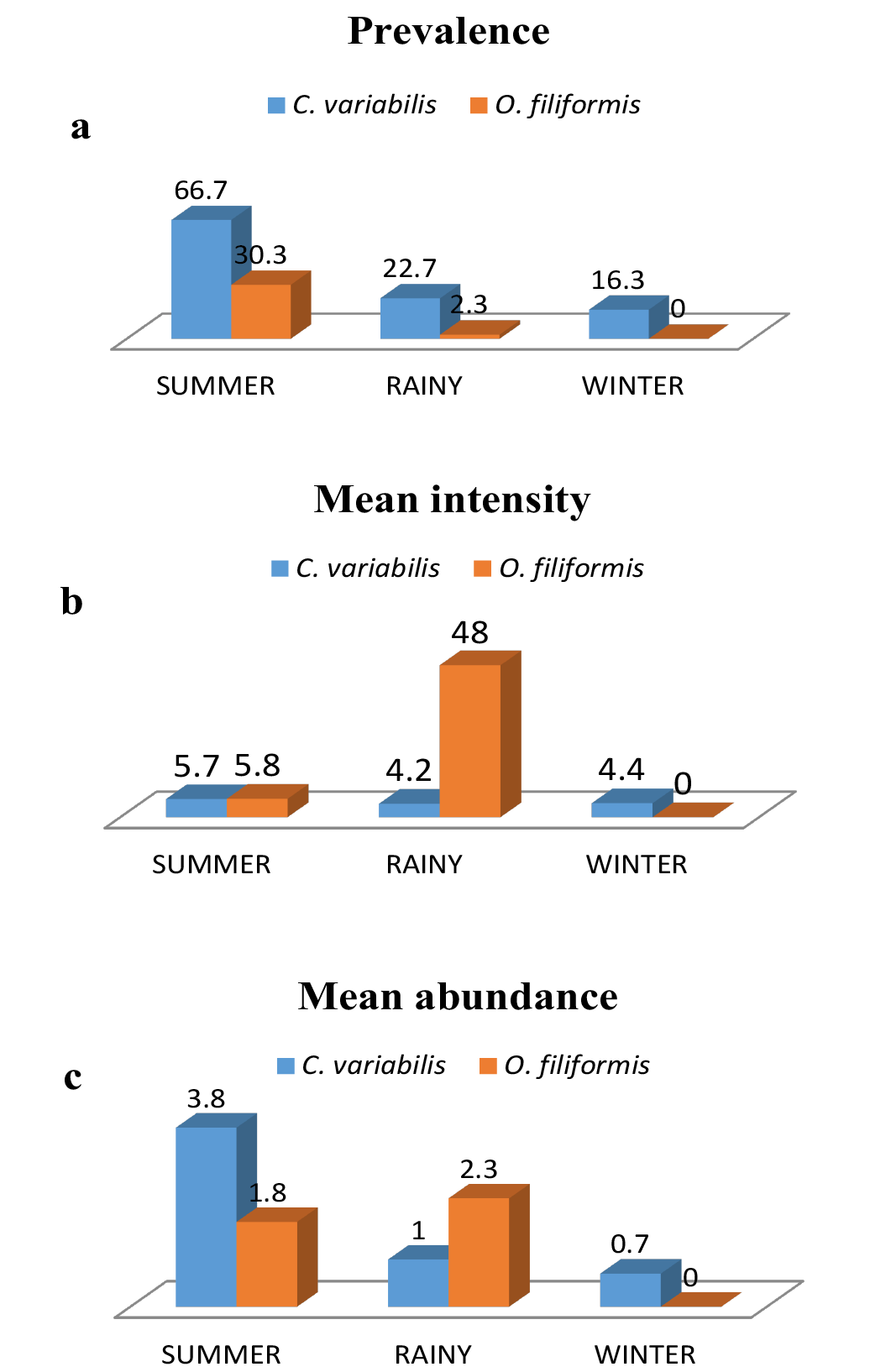
High prevalence was noted in the months of March to June and October for C. variabilis whereas O. filiformis showed highest prevalence in the month of April (Figure 1a). O. filiformis showed high intensity in September whereas in other months intensity was low to almost nil and abundance was high during June and September (Figure 1b and 1c). C. variabilis showed high intensity and abundance in October, however in the remaining months the infection was moderate to low (Figure 1b and 1c). Both the parasites showed highest prevalence in summer and lowest prevalence in winter. Intensity of infection was high in rainy season for O. filiformis and mean abundance was high in summer for C. variabilis while it was high in rainy season for O. filiformis (Figure 2a, 2b and 2c).
a) Prevalence; b) Mean intensity and c) Mean abundance
Locality is one of the crucial ecological factors which play a significant role in the occurrence of parasites. Locality refers to the spatial region or geographical position of the external environment from where the parasite is isolated (Bush et al., 1997; Chandra and Gupta, 2007). In the present study, the host, H. tigerinus were collected from three sites. Of the 113 hosts examined from Industrial Estate area, 35 frogs (30.9%) were parasitized with 165 C. variabilis with an intensity of 4.71 and 10 frogs (8.84%) were infected with 58 O. filiformis with an intensity of 5.8. Of the 12 frogs examined from University campus, only 3 frogs (25%) with 29 C. variabilis were infected and parasitization with O. filiformis was nil. Of the 5 frogs examined from Ramapuram village, only 1 frog (20%) was infected with both parasites (Table 2). In the present study, Site-A seems to be most preferred site for the parasite invasion than the other two sites due to various human activities which disturb the habitat and alter the host health status favouring the invasion of parasites.
a) Prevalence; b) Mean intensity and c) Mean abundance
Table 3: Organwise distribution of Nematode parasites in H. tigerinus (n=130)
|
Name of the parasite |
Stomach |
Intestine |
Rectum |
Total no. of parasites |
|
Cosmocercoides variabilis |
79 (39.69%) |
95 (47.73%) |
25 (12.56%) |
199 |
|
Oswaldocruzia filiformis |
- |
11 (10.37%) |
95(89.6%) |
106 |
|
Total no. of parasites in different organs (% of parasites in different organs) |
79 (25.9%) |
106 (34.7%) |
120 (39.34%) |
305 |
Table 4: Effect of Host size on the infection of Nematode parasites in H. tigerinus
|
Sl. No |
Size groups |
Class Intervals |
No. of parasites |
||
|
Both parasites |
C. variabilis |
O. filiformis |
|||
|
1 |
Group-I |
4-10 cm |
128 |
79 |
49 |
|
2 |
Group-II |
10-16cm |
130 |
77 |
53 |
|
3 |
Group-III |
16-22 cm |
47 |
43 |
43 |
|
Correlation Coefficient, r |
r = 0.00104 |
r = -0.0412 |
r = 0.062 |
||
Table 5: Infection of nematode parasites in males and females of H. tigerinus (Nm = 84; Nf= 46)
|
Name of the parasite |
Nmi |
Nfi |
Pam |
Paf |
Prm |
Prf |
MIm |
MIf |
MAm |
MAf |
χ2 – test at 5% level of significance, 1˚degree of freedom |
|
C. variabilis |
24 |
15 |
137 |
62 |
28.5 |
32.6 |
5.7 |
4.13 |
1.63 |
1.34 |
0.795 |
|
O. filiformis |
9 |
2 |
104 |
2 |
10.7 |
4.34 |
11.5 |
1 |
1.2 |
0.04 |
8.013 |
|
Both nematodes |
25 |
15 |
241 |
64 |
29.7 |
32.6 |
9.64 |
4.2 |
2.8 |
1.39 |
5.53 |
Nm: number of males examined, Nf: number of females examined; Nmi: number of infected males; Nfi: number of infected females; Pam and Paf: Total no. of parasites in males and females; Prm and Prf: prevalence of males and females, respectively; MIm and MIf: mean intensity of males and females; MAm and MAf: mean abundance of males and females.
Parasites are frequently found along the alimentary canal, rectum and intestine. The intestine is the most preferred spots of the infection and the parasite appears to maintain a commensalistic relationship with its host and as it does not pose any risk though present in abundant numbers (Noble, 1930; Reichenbach-klinke and Elken, 1965; Begum and Banu, 2012). The stomach, intestine and rectum of H. tigerinus were screened to record the number of nematode parasites. Rectum showed the maximum infection (39.34%) with both the nematodes followed by intestine (37.4%) and stomach (25.9%). C. variabilis showed infection in all the three organs with highest in intestine (47.73%) followed by stomach (39.69%) and rectum (12.56%) whereas O. filiformis showed highest infection in rectum (89.6%), least in intestine (10.37%) and nil in stomach (Table 3).
All the sampled hosts ranged between 4-18.5 cm (mean= 10.97±3.78) in total length. The average total snout-vent length of male (10.88±3.77 cm, n=46) and female (11.15±3.84 cm, n=84) frog in the sample were not significantly different (t= 0.34). Correlation coefficient ‘r’ was used to analyse possible relationship between host size and parasitic abundance. The coefficient value r=-0.0412 C. variabilis illustrates a very insignificant positive correlation between host size and number of parasites. However, r=0.062 shows there is no impact of host length on parasitization of O. filiformis (Table 4). The present study supports the view of Aho (1990), Sluys et al. (1994), Koyum (2012) and Hemalatha et al. (2015) who opined that parasitization depends on host-size to some extent.
On the other hand, Lees (1962), Cheng (1964), Kennedy and Lie (1974), Al-Barware et al. (1980), Ahmed and Begum (2006a, 2006b) and Begum and Banu (2012) reported that the parasitization of male hosts is relatively higher than the female hosts. Cheng (1964) proposed that the male hormones may favour the growth and survival of parasites whereas Kennedy and Lie (1974) suggested that female hormone estrogen might depress the level of parasitization. Of the 84 (64.6%) male frogs, 25 frogs (29.76%) showed infection with both parasites and 15 (32.6%) of the 46 (35.3%) female frogs were infected with both parasites. The calculated χ2 -value (5.53) at 5% level of significance and at 1° degree of freedom was found to be greater than the tabulated χ2 –value (3.84) which suggests that there is statistically significant difference in parasitic abundance between male and female hosts. Males show more parasite abundance than female hosts. There is no influence of host sex on the parasitization of C. variabilis (χ2 -value 0.795) but parasitization of O. filiformis (χ2 -value 8.01) was more in males than females (Table 5). The present study showed noticeable variations in seasonal occurrence and the infectivity of nematode parasites which might be due to host behaviour, feeding habits, biology of host, size, sex and diversity in climatic conditions.
ACKNOWLEDGEMENTS
The first author is grateful to University Grants Commission for providing the financial assistance as JRF and SRF under UGC-RGNF scheme (F1-17.1/2011-12/RGNF-SC-AND-5015).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest related to the work.
Author’s Contribution
Hemalatha Mannela, Srinivasa C Kalyan had collected the host samples and parasites and prepared the initial manuscript while Anuprasanna Vankara which is also the corresponding author, has framed and formatted the paper.
REFERENCES