The Journal of Advances in Parasitology
Research Article
An Overview of the Trichostorngyloidea
Qasim Ali1*, Imran Rashid2, Kamran Ashraf2, Zubair Shabbir3, Umer Chaudhry4
1Department of Parasitology, Gomal University Dera Ismail Khan, Khyber Pakhtoon Khah, Pakistan; 2Department of Parasitology, University of Veterinary and Animal Sciences Lahore, Punjab, Pakistan; 3Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan; 4University of Edinburgh, Royal (Dick) School of Veterinary Studies and Roslin Institute,Easter Bush Veterinary Centre, Midlothian, Scotland, EH25 9RG, UK.
Abstract | Among helminthes, the trichostrongyloidea consists of most significant parasites of ruminants, which are posing significant threats to livestock productivity, health and well-being. This brief commentary provides an overview of this important group of parasite to showcase the crucial roles these play in hosts.
Keywords | Trichostrongyloidea, Ruminants, Helminthes
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | June 26, 2019; Accepted | July 05, 2019; Published | April 25, 2019
*Correspondence | Qasim Ali, Department of Parasitology, Gomal University Dera Ismail Khan, Khyber Pakhtoon Khah, Pakistan; Email: [email protected]
Citation | Ali Q, Rashid I, Ashraf K, Shabbir Z, Chaudhry U (2019). An overview of the trichostorngyloidea. J. Adv. Parasitol. 6(2): 21-23.
DOI | http://dx.doi.org/10.17582/journal.jap/2019/6.2.21.23
Copyright © 2019 Ali et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastrointestinal (GI) round worms are causing hurdles in the way of soundness for both livestock and human beings (Sutherland and Leathwick, 2011). In human, diseases due to GI roundworms are the most universal all over the world. There are 3.5 billion cases were recorded around the world, among these 450 million are people who are gravely sick thus, the generally are kids, and around 125,000 deaths were recorded per annum (Stepek et al., 2006). For over 50 years, the quantity of cases of GI nematode infections has surged with the overall populace, to such an extent that more than half of the worldwide population is suffered by the six important GI roundworms species (Chan, 1997; Stepek et al., 2006; Ziegelbauer et al., 2012). Gastrointestinal nematode infections are plentiful and among the most expensive illnesses in the domesticated animals industry. Ordinary overviews demonstrate that GI nematode parasites charge the American dairy cattle industry higher than $2 billion for each annum in term of low yield and ascend in working expenditure (Redman et al., 2015). The connection of cattle and their GI roundworms parasites can bring about various biological reactions (loss of hunger, stomach pain, etc.) that adversely impact animal production and welfare. These reactions result from the direct devastative impact of the worms on animal body, and more secondary effect, for example, an potential clamp-down of immune responses unimportant to parasite antigens or immunopathology (Fox, 1997). GI nematodes can apply a few consequences for cows that can fundamentally hinder the general health and prosperity of the host, including direct impacts on the digestion and absorption of required nutrient, and indirect consequences for the host immune system that may make a reduced capacity react to different infectious agents or reduced efficiency from an inflated stimulation of the gut resistant system (Stromberg and Gasbarre, 2006). Animal illness produces extensive variety of biophysical and financial effects that might be both direct and indirect, and may change from much confined to worldwide issues such as loss of animals’ profitability, treatment costs, loss of farm profitability, troubling influence of human health and disturbing of human welfare (Murrell, 1991). The cost of parasitism in the Australian domesticated animal industries is considerable. Of the 4 chosen parasites, sheep roundworms inflicted the highest cost to the Australian domesticated animal industries (McLeod, 1995).
Classification and Life cycle of the Trichostorngyloidea
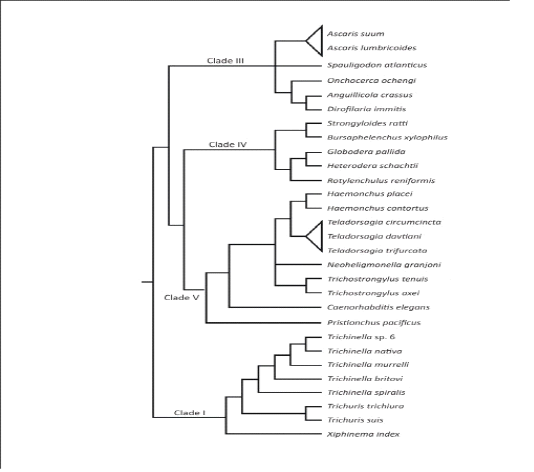
The phylum Nemathelminths has six branches, the nematodes form one of them, which contains parasite of veterinary importance.Nematodes are ordinarily called roundworms, due to their look of rounded body structure. These roundworms are additionally subdivided into order, superfamily, genus, species and a most essential divisions: bursate and non-bursate clusters due to the presence of bursa (Urquhart et al., 1996). Mostly individuals from the order Strongylida of nematoda class bursate group, which additionally subdivided into 4 diverse superfamilies: the trichostrongyloidea (contain most significant parasites of ruminants), the strongyloidea (contain numerous significant parasites of equine),the ancylostomatidae the (contain hookworms of the human and pets) and the metastrongyloidea (contain worms of lungs of pets animals) (Anderson et al., 1998). After the approach of molecular genetics, the evolutionary system has been built up on the basis of little subunit of DNA of the ribosomes which separate the parasitic roundworms hereditarily into five important clades I-V (Figure 2.1) (Gilabert and Wasmuth, 2013). The roundworms in the superfamily trichostongyloidea (Clade V) which are most essentialgastrointestinal (GI) parasites of ruminant livestock animals (Gilleard, 2013). The major GI nematode types of cattle in the superfamily trichostongyloidea are Haemonchusplacei, O.ostertagi, M.digitatus and T.axei present in the abomasum; S.papillosus,Cooperiaand Nematodirrus parasites are present in the small intestinal tract; and O.radiatum present in large intestinal tract (Fukumoto et al., 1990; Oku et al., 1987). Among every single above species,H. placei, C. oncophora and O.ostertagi are estimated the most predominant in bovine; especially the rate of infection of H. placei was very high in cattle.
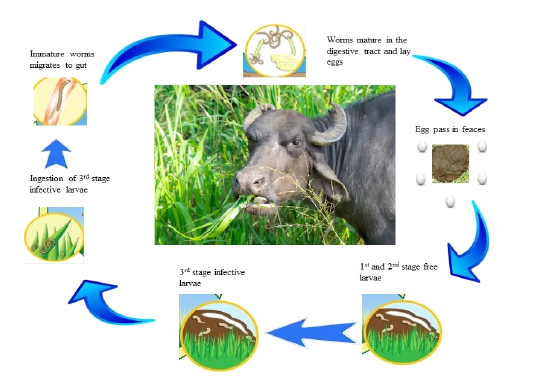
The life cycles of roundworm parasites are direct and extensively same in all parasites (see in Figure 2.1). On account of gastrointestinal roundworms, the stages of growth occur in the GI tract, where they lay extensive quantities of eggs by the fertilized females that are released from body through feces. Trichostrongyloidea eggs are incubate done to two days inside the dung and hatched into L1 stage larvae, which are further, transformed into the L2 and L3 stage larvae inside the dung. The L3 is the infective stage larvae, which relocate their position from dung to pasture, fodder, water reservoir and once L3ingested by the animal, molt into L4 larvae. After thatL4 is converted into adult in the GI tract. After mating with male, the adult female worms begin laying eggs at day 18.An amazing egg production of a fertilized female reaches to range between 5000– 15,000 eggs per day (Figure 2.2) (Zajac, 2006).
References