Research Journal for Veterinary Practitioners
Short Communication
Myocardial Fibrosis and Colisepticaemia in a Bornean Orangutan (Pongo pygmaeus)
Annas Salleh
Department of Veterinary Laboratory Diagnosis, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia.
Abstract | Myocardial fibrosis the most important cardiovascular disease (CVD) in great apes, especially in orangutan (Pongo sp.) and gorilla (Gorilla sp.). This article describe for the first time a case of mild myocardial fibrosis leading to septicaemia in a bornean orangutan (Pongo pygmaeus). A 24 year old bornean orangutan was reported with two episodes of inactivity and inappetence before its death. At necropsy, jaundice, severe pulmonary congestion and haemorrhages, pulmonary oedema and hepatomegaly were among the most important lesions observed. Histopathological examination revealed mild left ventricular myocardial fibrosis and haemosiderin-laden alveolar macrophages. Subsequent isolation of Escherichia coli from multiple organs confirmed the diagnosis of colisepticaemia.
Keywords | Cardiovascular disease, Colisepticaemia, Myocardial fibrosis, Orangutan, Pongo sp
Editor | Muhammad Abubakar, National Veterinary Laboratories, Islamabad, Pakistan.
Received | March 02, 2017; Accepted | March 26, 2017; Published | March 29, 2017
*Correspondence | Annas Salleh, Department of Veterinary Laboratory Diagnosis, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; Email: [email protected]
Citation | Annas S (2017). Myocardial fibrosis and colisepticaemia in a bornean orangutan (Pongo pygmaeus). Res. J. Vet. Pract. 5(1): 15-18.
DOI | http://dx.doi.org/10.17582/journal.rjvp/2017/5.1.15.18
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Annas Salleh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Orangutan (Pongo sp.) is one of the great apes (Hominidae), alongside the gorilla (Gorilla sp.), chimpanzees and bonobo (Pan sp.) and human (Homo sp.) (Prado-Martinez et al., 2013). Orangutan is an Asian species, and known to be native to Malaysia (Borneo) and Indonesia (Borneo and Sumatra) (Miles, 2007). There are two species of orangutan; the bornean orangutan (Pongo pygmaeus) and the sumatran orangutan (Pongo abelii). Despite extensive conservation effort, the orangutan is declared as critically dangered and listed in the IUCN red list (Ancrenaz et al., 2016; Singleton et al., 2016).
Cardiovascular disease (CVD) has been described in all species of great apes including human. One of the most common CVD in the non-human great apes includes myocardial fibrosis. In orangutan, heart diseases have been previously described but with very limited reports. This includes coxsakie virus B4 myocarditis (Miyagi et al., 1999) hypertensive heart disease and stroke (Weisenberg et al., 1991) cardiac fibrosis (Munson and Montali, 1990) chronic pericardial effusion (Robbins, et al., 2009) and congenital atrial septal defect (Greenberg et al., 1999).
It is known in both human and veterinary medicine that CVD may lead to wide variety of complication such as pulmonary thromboembolism, pulmonary oedema and septicaemia (LaRue and Murtaugh, 1990; Oudiz, 2007; Ishani et al., 2005). The present article describes for the first time a case of myocardial fibrosis leading to colisepticaemia and death of a bornean orangutan, with emphasis on the pathology of the case.
A carcass of a 24 year old female captive bornean orangutan was submitted to the necropsy laboratory. One month prior to its death, the orangutan has had a history of being inactive and inappetence. It was treated with potentiated amoxicillin (Clavamox®) and ketoprofen (Ketofen®) and the clinical signs resolved after 5 days. 15 days later, the animal was inactive and inappetence again, and was treated with meloxicam (Metacam®). One day after the treatment, haemoptysis and left unilateral serosanguineous nasal discharge was observed. The orangutan was found dead after five days, and the carcass was immediately submitted to the necropsy laboratory.
Upon examination, the orangutan was of good body condition, with normal skin and hair. The conjunctiva and eyelids were moderately jaundiced. Bilateral dried
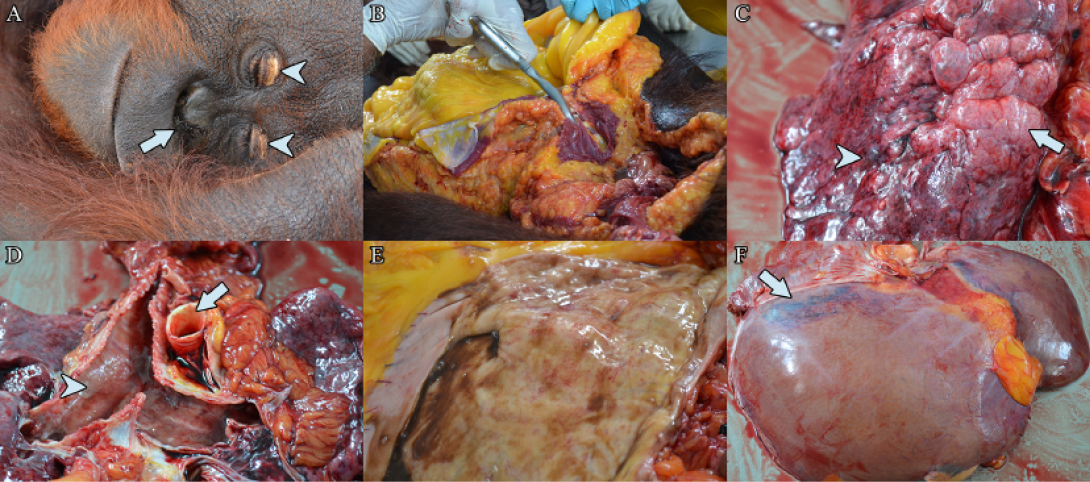
Figure 1: (a) Bilateral dried haemorrhagic nasal discharge (arrow), with bilateral yellowing of the eyelid (arrow heads). (b) Severe jaundice of the subcutaneous and visceral fat. (c) Pulmonary emphysema (arrow), with areas of pulmonary petechiation (arrow head). (d) Yellowing of the aorta (arrow) with presence of frothy serosanguineous exudate along the congested trachea (arrow head). (e) Mild haemorrhage on the stomach mucosa. (f) Yellow discoloration and generalised hepatomegaly, with hepatic haemorrhage (arrow)
haemorrhagic nasal discharge was observed (Figure 1a) with severe jaundice of the subcutaneous and visceral adipose tissues (Figure 1b). In the respiratory tract, severe pulmonary oedema was observed in the trachea and lungs (Figure 1c). Pulmonary petechiation and focal emphysema was observed on the left lung lobe. No gross lesion was noted upon examination of the heart. The tunica intima of the aorta was severely jaundiced (Figure 1d). The stomach and intestine were empty, with haemorrhagic gastroenteritis (Figure 1e). Mild hepatic lipidosis, generalised hepatomegaly with sub-capsular hepatic haemorrhages was observed (Figure 1f). Samples of lungs, liver, kidneys, spleen, and heart were collected for histopathology and bacterial isolation and identification.
Microscopically, examination of the lungs revealed severe pulmonary oedema, severely congested capillaries, with evidence of disseminated intravascular coagulation (DIC) (Figure 2a), severe pulmonary atelectasis (Figure 2a) and multifocal areas of ruptured interalveolar septa. Severe pulmonary haemorrhage, with moderate to severe infiltration of neutrophils and macrophages were noted. Numerous haemosiderin-laden laden macrophages was observed (Figure 2b). In the heart, there were areas of the mild replacement of myocardium by fibrous tissue in the left ventricle without infiltration of inflammatory cells (Figure 2c). In the liver, most hepatocytes were swollen with generalised hepatic lipidosis (Figure 2d). In the spleen, splenic hypocellularity was observed along with the presence of mild splenic haemosiderosis. In the kidney, generalised tubular necrosis and haemorrhage was observed. A pure culture of the same bacteria colonies were obtained from all organs upon subjected to bacterial isolation. Gram staining of these colonies revealed Gram-negative rod, with biochemical test suggestive of Escherichia coli.
Based on the histopathologic findings of presence of fibrous tissue in the myocardium, and haemosiderin-laden macrophages in the lungs, the case was diagnosed as myocardial fibrosis, complicated by E. coli infection leading to colisepticaemia, Death acutely transpired following colisepticaemia.
CVD is known to be a major cause of mortality in great apes especially in captive management. A survey conducted among gorilla in the Association of Zoos an Aquaria, North America Species Survival Plan, it was concluded that CVD is the primary, or an important contributory cause of death, accounting up to 41% (Meehan and Lowenstine, 1994). Other than that, it was also observed that CVD is an important disease condition in other great apes, contributing to 20% death of orangutans (Lowenstine et al., 2008), 38% death of chimpanzees (Gamble et al., 2004) and 45% death of bonobos (Meehan and Lowenstine, 1994).
Currently, few types of CVD has been identified in apes, with fibrosing cardiomyopathy (or also known as myocardial fibrosis) being the most common (Miller and Fowler, 2011). Fibrosing cardiomyopathy has been described as idiopathic replacement of myocardial by fibrous tissues. This is accompanied with atrophy and hypertrophy of cardial myocytes. Inflammation of the myocytes is usually absent or minimal. Furthermore, no apparent aetiology or
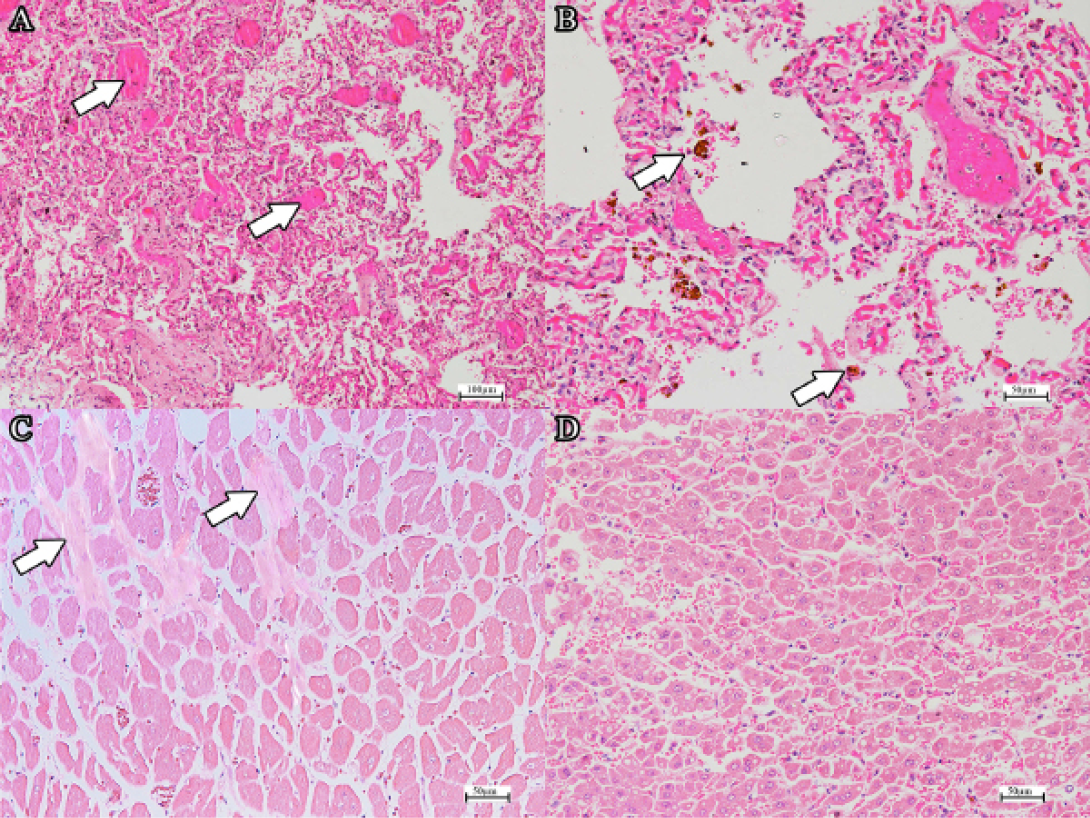
Figure 2: (a) DIC in the pulmonary capillaries (arrows) (H and E, bar=100µm). Note the extensive atelectasis. (b) Haemosiderin-laden alveolar macrophages (H and E, bar=50µm). (c) Mild fibrosis of the myocardium (arrow) (H and E, bar=50µm). (d) Generalised mild to moderate hepatic lipidosis (H and E, bar=50µm).
diseases has been linked to fibrosing cardiomyopathy. It is an end-stage lesions, with no specific aetiology or pathogenic mechanism. It develop as a consequences of a chronic myocardial injury, such hypoxia, ischemia, necrosis and inflammation (Miller and Fowler, 2011). However, in orangutans and other great apes, these causes are not fully understood.
In this particular case, the histopathologic lesion of the heart presented only with mild myocardial fibrosis. Perhaps, this is because the orangutan was still in the early susceptible age (Munson and Montali, 1990). Plus, myocardial fibrosis is known to be a chronic manifestation upon non-specific myocardial injury (Miller and Fowler, 2011). Regardless of the type of myocardial fibrosis (scarring myocardial fibrosis or interstitial myocardial fibrosis), the outcome and manifestation are similar. Heart diseases is known to be a risk factor contributing to respiratory infection (Thomsen et al., 2008) whereby in this case, the mild left ventricular myocardial fibrosis eventually lead to pulmonary infection and death of the orangutan. The myocardial fibrosis lead to inefficient pumping of the left ventricle, due to the non-contractile nature of this collagenous tissue (Lammey et al., 2008) backflow of blood into the lungs, pulmonary congestion, leading to per diapedesis pulmonary haemorrhage, pulmonary oedema, and pulmonary haemosiderosis. This respiratory involvement resulted in the orangutan prone to secondary respiratory infection by E. coli which in turn leads to colisepticaemia. Respiratory infection in orangutan has been studied, with involvement of E. coli in 65% of the cases (Zimmermann et al., 2011). Previous retrospective study among chimpanzee found that 8.3% of the animal were septicaemic as a complication of minimal to mild myocardial fibrosis (Lammey et al., 2008). The episodes of inappetence brought about the findings of hepatic lipidosis and hepatomegaly, due to excessive mobilisation of fat depot for energy production (Rouvinen-Watt, et al., 2010; Nieminen, et al., 2009). This in turn, leads to hepatic jaundice characterised by severe yellowing of the fat tissues. In many cases of CVD of great apes, the animals are usually asymptomatic, or showing non-specific clinical signs such as inactivity and inappetence. Thus, thorough routine medical examination especially for orangutan aging more than 20 years old is warranted for earlier diagnosis of CVD.
CVD is the most important disease leading to death of great apes, including the orangutan. CVD may lead to death of orangutan by other complication, such as septicaemia. Although myocardial fibrosis is mild, the animal may succumb due to other complications. At necropsy, diagnosis of myocardial fibrosis is straight forward with the aid of histopathology. However, clinical diagnosis requires thorough clinical examination due to the vague clinical signs.
REFERENCES






