Journal of Animal Health and Production
Research Article
Effects of Harvesting Time on Tannin Biological Activity in Sambiloto (Andrographis paniculata) Leaves and In vitro Diet Digestibility Supplemented with Sambiloto Leaves
Dadang Priyoatmojo, Yunida Maharani, Dedi Ansori, Shintia Nugrahini Wahyu Hardani, Afi Candra Trinugraha, Tri Handayani, Wahidin Teguh Sasongko, Teguh Wahyono*
Department of Agriculture, Center for Isotopes and Radiation Application, National Nuclear Energy Agency of Indonesia (BATAN), Lebak Bulus Raya St. No. 49, Cilandak, South Jakarta, 12440, Indonesia.
Abstract | This study investigated the effect of harvesting time on concentration and biological activity of tannin in Andrographis paniculata (A. paniculata) leaves and determined the in vitro rumen fermentation and digestibility of diet supplemented with A. paniculata leaves to evaluate its role as herbal supplement in ruminant’s feed. A. paniculata leaves were harvested at two time points i.e., before and after flowering phase and the samples were dried to analyze concentration and biological activity of tannin. Rice straw was used as basal diet to carry out in vitro digestibility trial. Four treatments were used including B consisting basal diet only, BBF and BAF each consisting basal diet supplemented with 1% A. paniculata leaves before and after flowering phases, respectively, and BBAF supplemented with 0.5% A. paniculata leaves before and 0.5% after flowering phase. Each treatment was carried out in five replications. Results revealed that contents (mg/g) for total tannin (20.40±1.06 vs 19.33±1.19; P<0.05) and condensed tannin (8.44±0.17 vs 5.47±0.30; P<0.01) were greater in leaves harvested after flowering compared with before flowering phase, however, no significant difference (P > 0.05) on biological activity of tannins was observed between the two flowering phases. The in vitro gas production was not influenced (P > 0.05) by the supplementation of A. paniculata leaves before and after flowering phase. A. paniculata supplementation also did not influence (P > 0.05) in vitro organic matter digestibility (IVOMD) and total volatile fatty acids (TVFA) values. It was concluded that the supplementation of A. paniculata leaves before and after flowering phase in the diet does not affect the digestibility.
Keywords | Andrographis paniculata, Biological activity, Flowering phase, In vitro digestibility, Tannin
Received | July 21, 2021; Accepted | August 08, 2021; Published | October 01, 2021
*Correspondence | Teguh Wahyono, Department of Agriculture, Center for Isotopes and Radiation Application, National Nuclear Energy Agency of Indonesia (BATAN), Lebak Bulus Raya St. No. 49, Cilandak, South Jakarta, 12440, Indonesia; Email: [email protected]
Citation | Priyoatmojo D, Maharani Y, Ansori D, Hardani SNW, Trinugraha AC, Handayani T, Sasongko WT, Wahyono T (2021). Effects of harvesting time on tannin biological activity in sambiloto (andrographis paniculata) leaves and in vitro diet digestibility supplemented with sambiloto leaves. J. Anim. Health Prod. 9(4): 425-434.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.4.425.434
ISSN | 2308-2801
Copyright © 2021 Wahyono et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
A. paniculata or “King of Bitters” is an annual herbaceous plant that extensively cultivated in Asia and some parts of Europe (Joselin and Jeeva, 2014). In Indonesia, A. paniculata is also known as Sambiloto, commonly used a medicinal plant by the rural communities (Roosita et al., 2008). The major phytochemical compounds in the plant is andrographolide, however, there are many other active compounds in the form of alkaloids, phenols, tannins, flavonoids and saponins (Nagajothi et al., 2018; Sithara et al., 2016). As a medicinal plant, it possesses antibacterial, antioxidant, anti-inflammatory, antiprotozoal, nematicidal and immunomodulatory activities (Joselin and Jeeva, 2014). It also has antiviral and immunostimulant activity in animals (Nagajothi et al., 2018). Therefore, it can be used as an appropriate feed supplement to improve fermentation and ruminal diet digestibility in ruminants. It is
Table 1. Feed composition of the experimental diets
| Experimental diets | ||||
| Ingredient (% DM) | B |
BBF |
BAF |
BBAF |
| Rice straw | 60.00 | 60.00 | 60.00 | 60.00 |
| Pollard | 16.50 | 16.50 | 16.50 | 16.50 |
| Bakery wastes | 20.00 | 20.00 | 20.00 | 20.00 |
| Mineral mix | 2.00 | 2.00 | 2.00 | 2.00 |
| Urea | 1.00 | 1.00 | 1.00 | 1.00 |
|
CaCO3 |
0.50 | 0.50 | 0.50 | 0.50 |
| APBF | 0.00 | 1.00 | 0.00 | 0.50 |
| APAF | 0.00 | 0.00 | 1.00 | 0.50 |
APBF: A. paniculata leaves before flowering phase; APAF: A. paniculata leaves after flowering phase; B: basal diet; BBF: basal diet + 1% A. paniculata leaves before flowering phase; BAF: basal diet + 1% A. paniculata leaves after flowering phase; BBAF: basal diet + 0.5% A. paniculata leaves before and 0.5% after flowering phase
necessary to know the concentration of tannins at different harvesting times in A. paniculata and its effects on in vitro fermentation and digestibility of the diet. Low to moderate concentrate of tannins has beneficial effect include protection of dietary protein from ruminal degradation, modulate ruminal biohydrogenation and reduce enteric methane emission (Frutos et al., 2004).
The inclusion of A. paniculata in ruminant’s diet have been reported by previous studies. Andrographis paniculata supplementation in the diets reduced ruminal protein breakdown and improved in vitro digestibility (Yusuf et al., 2012). It has been shown that A. paniculata herb supplementation in diet based on urea-treated rice straw can be fed to ruminant without impairing their performance (Roslan et al., 2016). Herb supplementation of A. paniculata leaf powder improved nutrient digestibility in goats (Yusuf et al., 2017). Moreover, Yusuf et al. (2014) demonstrated that A. paniculata inclusion has significantly improved growth performance, carcass and meat yield of Boer goats.
The leaves of A. paniculata contains higher total polyphenols and total tannin than the stem and roots (Yusuf et al., 2012). We hypothesized that the effectiveness of A. paniculata leaves as herb supplement may be influenced by the harvesting time. However, information regarding the effects of harvesting time on tannin concentration in leaves of A. paniculata and its biological activity and its effectiveness in rumen fermentation is very scarce. Thus, the objective of present study was to investigate the effect of harvesting time (before or after flowering phase) on tannin concentration and biological activity of A. paniculata leaves. The second objective was to evaluate the in vitro digestibility of diet supplemented with A. paniculata leaves.
MATERIALS AND METHODS
Raw materials and basal diet preparation
Andrographis paniculata was obtained from field laboratory, Center for Isotopes and Radiation Application (CIRA), National Nuclear Energy Agency of Indonesia (BATAN). Andrographis paniculata leaves were harvested before and after flowering phase. Leaves samples were placed in individual paper bags and dried at 60 oC for 72 h. Dry samples were ground with hammer mill into 1 mm particle size. Rice straw was obtained from rice field, CIRA, BATAN. The whole rice plant was harvested approximately 10 cm above the ground and straw was collected by removing the panicles. Rice straws were placed in paper bags and dried at 60oC for 72 h. Samples were ground into 1 mm particle size and used for chemical analysis and diet formulation. Pollard, bakery wastes, mineral mixture, urea and CaCO3 were obtained from local market. Basal diet composition represent in Table 1.
Chemical analysis
Total phenols, total tannins and condensed tannin contents in A. paniculata leaves were determined according to the method of Cindrić et al. (2011) and Makkar (2003). Ash, organic matter (OM), crude protein (CP) and ether extract (EE) contents were determined following the method of AOAC (2005). Fibrous components, such as neutral detergent fiber (NDF), acid detergent fiber (ADF) and acid detergent lignin (ADL) in raw materials were measured by the methods of Van Soest et al. (1991).
Experimental Design
Two trials were conducted in this study:
(I) Biological activity of tannin in A. paniculata leaves
AP BF - : A. paniculata leaves before flowering phase
AP BF + : AP BF + 400 mg polyethylene glycol (PEG)
AP AF - : A. paniculata leaves after flowering phase
AP AF + : AP AF + 400 mg polyethylene glycol (PEG)
Polyethylene glycol (Sigma-Aldrich) 6000 was used as tannin-binding agents (Getachew et al., 2000).
(II) Effects of A. paniculata leaves supplementation on in vitro rumen fermentation of basal diets
B: basal diet
B BF: basal diet + 1% A. paniculata leaves before flowering phase
B AF: basal diet + 1% A. paniculata leaves after flowering phase
B BAF: basal diet + 0.5% A. paniculata leaves before and 0.5% after flowering phase
In vitro evaluation was carried out in five replications.
In vitro Evaluation
In vitro evaluation for biological activity of tannin and supplementation of A. paniculata leaves were determined according to in vitro gas production technique (Menke and Steingass, 1988). Feed samples (200 mg) were weighed into 100 ml glass syringes (Fortuna, Labortechnik, Germany) before the rumen-buffer fluid was added. Rumen liquor inoculant was collected from three local cattle (average 300 kg live weight) that were slaughtered at local abattoir in Pamulang, South Tangerang, Indonesia. Rumen fluids were homogenized in a laboratory blender and strained through four layers of cheesecloth, thus mixed into buffer solution (1:2, v/v). All handlings were carried out under CO2 flushing/anaerobic condition. The rumen-buffer (40 ml) was filled into glass syringes and incubated into water bath at 39 oC. After 3, 6, 9, 12, 24, 48 and 72 h, cumulative gas production was recorded. Exponential Ørskov equation (Ørskov and Mcdonald, 1979) was used to determine kinetics gas characteristics, as follows: P = a + b(1 – e-ct), where P represents gas production at t time, a is cumulative gas production from soluble fraction, b is cumulative gas production from insoluble fraction and c is the rate of gas production. The gas production from soluble fraction (GPSF) and insoluble fraction (GPNSF) was calculated according to Van Gelder et al. (2005), as follows:
GPSF (ml)= (gas production at 3 h x 0.99 x 5) – 3
GPNSF (ml)= (1.02 x ((gas production at 24 h x 5) – (gas production at 3 h x 5))) + 2
In vitro organic matter digestibility, metabolizable energy and total volatile fatty acids after 24 h incubation were calculated according to Menke et al. (1979) and Getachew et al. (2000), respectively, as follows:
IVOMD (%)= 4.88 + (0.889 x gas production at 24 h) + (0.45 x CP) + (0.0651 x ash)
EM (MJ/kg DM)= 2.20 + (0.136 x gas production at 24 h) + (0.057 x CP)
TVFA (mmol) = (0.0239 x gas production at 24 h) – 0.0601
Data TVFA were converted to mM
Biological activity of tannin was calculated based on the increase in in vitro gas production with or without PEG addition at 72 h incubation time. After 72 h incubation, the contents of syringes (approximate 20 ml) were transferred into centrifuge tubes and centrifuged for 15 min at 23.000 rpm (4oC). The supernatant was analyzed for NH3-N and pH values.
Statistical Analysis
Experimental data were analyzed using the one way analysis of variance (ANOVA). Means comparison between treatments were tested using Duncan’s multiple range test. Significant levels was accepted at P<0.05.
RESULTS
Concentration of nutrients, total phenols, total tannins and condensed tannin of A. paniculata leaves
The results of nutrient analysis of A. paniculata and experimental diets for Trial II were presented in Table 2. The contents of ash, CP, EE, NDF and ADF were higher in A. paniculata before flowering than A. paniculata after flowering. Conversely, the content of OM (%) in A. paniculata after flowering was slightly higher than before flowering. Total phenols, total tannins and condensed tannin of A. paniculata leaves were shown in Figure 1. The leaves of A. paniculata after flowering were higher in total tannins (20.40±1.06 mg/g TAE; P<0.05) and condensed tannin (8.44±0.17 mg/g LE; P<0.01) as compared to A. paniculata before flowering. Different harvesting time had no significant effect on total phenols concentration (mg/g) in A. paniculata leaves.
Biological activity of tannins in A. paniculata leaves
The biological activity of tannins in A. paniculata leaves was presented in Figure 2. The in vitro gas productions were greater (P<0.05) in the A. paniculata leaves after flowering than before flowering phase. The pattern of in vitro gas production is also increase after A. paniculata added with PEG (P<0.05). The treatment of APAF after PEG addition had greater (P<0.05) cumulative gas production than other treatments. However, after PEG addition, there was no significant differences on biological activity of tannins between two flowering phase treatments (Figure 3).
Table 2: The nutrient contents of the experimental diets and A. paniculata leaves
| % Dry matter | |||||||
| Ingredient (g/kg) | OM | Ash | CP | EE | NDF | ADF | Hemicellulose |
| Rice straw | 74.93 | 25.07 | 4.34 | 7.46 | 69.17 | 46.95 | 22.22 |
| Pollard | 91.82 | 8.18 | 17.00 | 2.07 | 49.12 | 16.93 | 32.19 |
| Bakery wastes | 77.39 | 22,61 | 28.40 | 0.85 | 67.09 | 43.79 | 23.30 |
| Mineral mix | 2.43 | 97.57 | - | - | - | - | - |
| Urea | - | - | - | - | - | - | - |
|
CaCO3 |
2.02 | 97.98 | - | - | - | - | - |
| APBF | 85.68 | 14.32 | 24.63 | 2.22 | 30.25 | 11.71 | 18.54 |
| APAF | 87.97 | 12.03 | 21.26 | 0.98 | 24.91 | 9.29 | 15.62 |
APBF: A. paniculata leaves before flowering phase; APAF: A. paniculata leaves after flowering phase; OM: organic matter; CP: crude protein; EE: ether extract; NDF: neutral detergent fiber; ADF: acid detergent fiber.
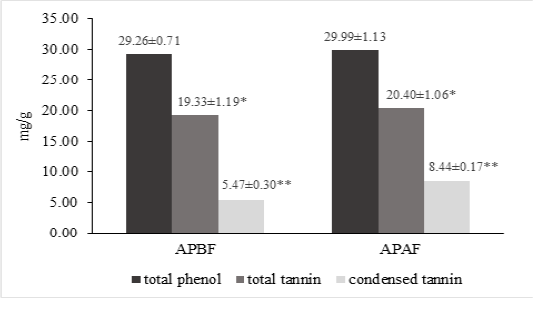
Figure 1: Concentrations (mg/g) of total phenols (gallic acid equivalent), total tannins (tannic acid equivalent) and condensed tannin (leucocyanidin equivalent) of A. paniculata leaves before and after flowering phase. *P<0.05; **P<0.01. APBF: A. paniculata leaves before flowering phase; APAF: A. paniculata leaves after flowering phase.
In vitro gas characteristics of diets containing A. paniculata leaves
The in vitro gas production and characteristics of diet containing A. paniculata leaves as herbal supplementation were listed in Table 3. Supplementation of 1% A. paniculata, both before and after flowering phase, did not reduce the cumulative gas production. The in vitro gas production also not influenced by the supplementation of 0.5% A. paniculata leaves before and 0.5% after flowering phase. The average optimum gas production (a+b) was significantly (P<0.05) higher in BBAF treatment than BAF and BBF groups. The mean rate of gas production (c) and GPSF values were not affected by A. paniculata supplementation. The BBAF treatment had higher GPNSF value than BAF group (P<0.05).
In vitro organic matter digestibility and rumen fermentation product of diets containing A. paniculata leaves
The in vitro organic matter digestibility and rumen fer
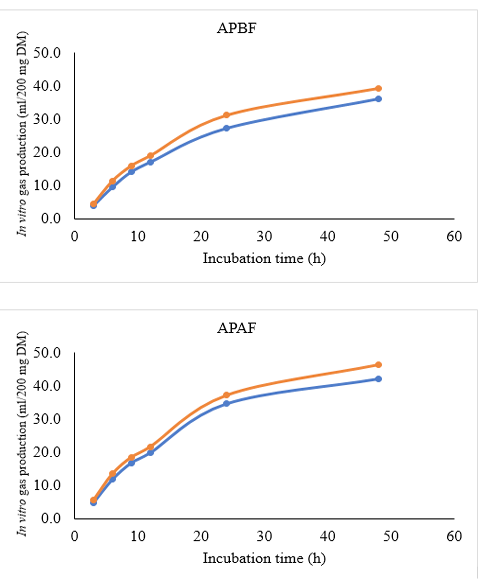
Figure 2: In vitro gas production of A. paniculata leaves before (o) and after (o) PEG addition. APBF: A. paniculata leaves before flowering phase; APAF: A. paniculata leaves after flowering phase
mentation characteristics of diets containing A. paniculata leaves as herbal supplement were presented in Table 4. Results demonstrated that the pH and NH3 concentration did not differ significantly across the treatments. A. paniculata supplementation treatments also did not influence IVOMD, ME and TVFA values. However, BBAF treatment had greater IVOMD, ME and TVFA values than BBF and BAF groups (P<0.05), nevertheless, there was no significant difference with control group (B treatment).
Table 3: Differences in the in vitro gas characteristics of diets containing A. paniculata leaves
| Treatment | Cumulative gas production (ml/200 mg DM) | Gas characteristics | ||||||||
| Incubation time (h) | ||||||||||
| 3 | 6 | 9 | 12 | 24 | 48 | a+b | c | GPSF | GPNSF | |
| B |
7.40± 0.92 |
17.38± 1.20ab |
22.63± 1.14ab |
25.95± 1.55ab |
35.06± 0.94ab |
40.54± 1.21bc |
40.45± 1.11ab |
0.094± 0.009 |
33.64± 4.54 |
143.08± 5.16ab |
| BBF |
6.71± 0.46 |
15.92± 0.65a |
21.16± 0.67a |
24.20± 0.87a |
33.94± 0.57a |
39.08± 0.57ab |
39.32± 0.47a |
0.089± 0.003 |
30.19± 2.29 |
140.91± 0.73ab |
| BAF |
7.57± 1.83 |
16.59± 1.67ab |
21.42± 1.79a |
24.36± 1.57a |
33.90± 1.46a |
38.84± 1.66a |
39.44± 1.35a |
0.088± 0.005 |
34.45± 9.04 |
136.32± 8.37a |
| BBAF |
8.11± 0.56 |
18.10± 0.63b |
23.26± 0.96b |
26.52± 1.09b |
36.42± 1.03b |
41.68± 1.07c |
41.82± 1.09b |
0.091± 0.006 |
37.14± 2.76 |
146.38± 3.22b |
B: basal diet; BBF: basal diet + 1% A. paniculata leaves before flowering phase; BAF: basal diet + 1% A. paniculata leaves after flowering phase; BBAF: basal diet + 0.5% A. paniculata leaves before and 0.5% after flowering phase; a+b: optimum gas production; c: the rate of gas production; GPSF: gas production from soluble fraction; GPNSF: gas production from non soluble fraction; means with the different superscripts in the same column are significantly different (P<0.05).
Table 4: Differences in the in vitro organic matter digestibility and rumen fermentation product of diets containing A. paniculata leaves
| Treatment | IVOMD | ME | pH |
NH3 |
TVFA |
| (%) | (MJ/kg DM) | (mg/100 ml) | (mM) | ||
| B |
47.89±1.08bc |
7.67±0.13ab |
6.59±0.13 | 5.53±0.16 |
19.45±0.56ab |
| BBF |
46.59±0.51ab |
7.52±0.08a |
6.68±0.08 | 5.49±0.43 |
18.78±0.34a |
| BAF |
46.38±1.47a |
7.51±0.20a |
6.51±0.14 | 5.17±0.15 |
18.75±0.87a |
| BBAF |
48.91±0.95c |
7.85±0.14b |
6.57±0.12 | 5.35±0.36 |
20.26±0.62b |
B: basal diet; BBF: basal diet + 1% A. paniculata leaves before flowering phase; BAF: basal diet + 1% A. paniculata leaves after flowering phase; BBAF: basal diet + 0.5% A. paniculata leaves before and 0.5% after flowering phase; IVOMD: in vitro organic matter digestibility; ME: metabolisable energy; NH3: ammonia; TVFA: total volatile fatty acids; means with the different superscripts in the same column are significantly different (P<0.05).
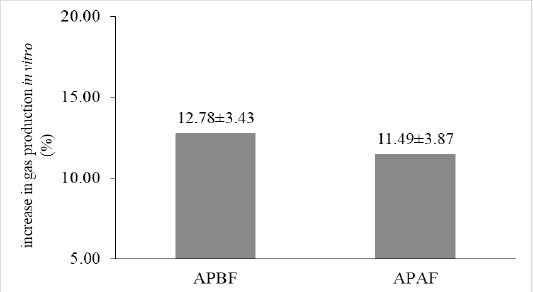
Figure 3: Biological activity of tannins in A. paniculata leaves before and after flowering phase. APBF: A. paniculata leaves before flowering phase; APAF: A. paniculata leaves after flowering phase.
DISCUSSIONS
Phenol characteristics and biological activity of tannin in A. paniculata leaves (before and after flowering phase)
Phenol is one of the bioactive compounds contained in A. paniculata which has received considerable attention in pharmacological field (Prabha and Anil, 2019) due to their antioxidant attributes (Chang et al., 2019). A. paniculata has been widely studied for its use as a feed supplements for ruminants Yusuf et al. (2012); Yusuf et al. (2014); Roslan et al. (2016); Wang et al. (2018); Yusuf et al. (2017) and Yusuf et al. (2018), however, there is limited information on the different harvesting stages of leaves. Our study investigated the differences in phenolic compounds which might have influence on the digestibility of basal diet. Total phenol compounds concentration in present study ranged between 29.26 – 29.99 mg/g GAE which was lower than previous study (30.80 mg/g) reported by Yusuf et al. (2012), although they did not evaluate the effect of harvesting time. Similarly, the values for total phenols compounds (11.62 – 12.45 mg/g) in A. paniculata leaves reported by Nagajothi et al. (2018) were lower compared to our study. Variations in phenolic concentrations depend on specific agro-ecosystem conditions such as temperature, altitude and precipitation (Dragović et al., 2020). Naumann et al. (2018) also demonstrated that the relationship between phenolic activity and maturity of herbal plants is species-specific.
Our study demonstrated that there are no significant effect on total phenols concentration (mg/g) in A. paniculata leaves between two harvesting stages. The present results are in agreement with those presented by Engin and Mert (2020) where the changes in total phenols compounds of “Nero” aronia berry cultivar were not significant over the harvest period. Furthermore, it was explained that the phenolic compounds started to increase after the flowering phase. In contrast, Kamaruzaman et al. (2020) observed that accumulation of phenolic contents in Piper crocatum was affected by harvesting time. Besides, Ahmed et al. (2020) also showed that the content of phenolic compounds was associated with maturity stages in Citrus paradise fruits. The difference in results may be due to the different climatic and environmental conditions, pre- and post-harvest factors such as species and cultivars and technical conditions (storage, drying process, transportation and extraction method) (Dragović et al., 2020; Kamaruzaman et al., 2020; Engin and Mert, 2020).
Tannins and condensed tannin compounds of A. paniculata leaves varied from 19.33 to 20.40 mg/g and 5.47 to 8.44 mg/g, respectively. The values for tannins were greater in the present study than that 5.71 to 6.37 mg/g reported by (Nagajothi et al., 2018). The values for condensed tannins also were greater in the present study than that 1.9 and 2.0 mg/g reported by Yusuf et al. (2012) and Yusuf et al. (2018), respectively. Dragovic et al. (2020) stated that plants constantly face environment stresses during their life cycle, thus it will be resulting in the differences of secondary metabolites biosynthesis. The increase in tannins and condensed tannin with growth stage especially after flowering phase are consistent with the results for sorghum grain and aronia berry (Engin and Mert, 2020; Wahyono et al., 2019). This may be due to the natural mechanisms of plant protection. In plant defense mechanism, the production of secondary metabolites such as phenolic and tannin increases to protect the maturation process of grain (Dragović et al., 2020; Wahyono, Astuti, et al., 2019). Phenolic contents (condensed tannin and phenolic acid) of persimmon leaves were significantly related to their antioxidant activities (Chang et al., 2019). Calabro et al .(2012) reported that condensed tannin compound in sainfoin was influenced by the phenological stage.
Biological activity of tannins are a measure of the tannin compounds produced by herbal plants that demonstrate binding and precipitating protein from plant itself. Polyethylene glycol (PEG) has a high affinity, bind tannins, and become unable to react with protein. An increase of in vitro gas production after PEG addition is a measure of the biological activity of tannins (Jayanegara et al., 2009; Kisworo et al., 2017). Although, there was no significant differences seen in biological activity between two treatment groups, the increase in in vitro gas production shown by APBF was numerically higher compared to APAF. However, APBF had lower tannins and condensed tannin compounds (Figure 1). Rodriguez et al. (2015) demonstrated that the response to increasing values after PEG addition is not linear but exponential, this explains that it is not related with the amount of tannins but specifically with their reactivity. The effects of tannins on nutrient digestibility related to the complexes formation between tannins and other components on plants (Calabrò et al., 2012). The biological activity of tannins also depends on their structure and the nature of protein on diets (Muetzel and Becker 2006). The increase in gas production in presence of PEG could be due to an increase in the available nutrient from leaves after binding tannins activity (Calabrò et al., 2012). Irrespective of the PEG addition, APBF treatment had lower in vitro gas production than APAF. The high fiber fraction (NDF and ADF) in A. paniculata before flowering phase (Table 2) may be the reason of low in vitro gas production. The variation in gas production characteristics was influenced by the differences of structural carbohydrates compounds (Wahyono et al., 2021; Wahyono et al., 2019). High soluble carbohydrate source and more amino acids are taken up into rumen microbial growth, thus increased fermentation activity with higher gas production parameters (Calabrò et al., 2012).
Effects of A. paniculata leaves supplementation on in vitro rumen fermentation
Gas production from fermentation process is the results of the degradability of carbohydrate compounds and the end products of microbial fermentation in the rumen (Kisworo et al., 2017). Although, there was no significant difference seen between basal diet and A. paniculata supplementation, the cumulative in vitro gas production shown by BBF and BAF was numerically lower compared to control (B). Apparently, this decrease was related to an inhibitory mechanism by secondary metabolites in A. paniculata leaves. Interaction between tannins and nutrient components such as protein and carbohydrates (non-structural and structural) could influence the decrease in gas production (Jayanegara et al., 2015). Kisworo et al. (2017) reported that secondary metabolites may inhibit fermentation of substrates due to its antimicrobial characteristics. Furthermore, the ability of rumen microbial to degrade the nutrient compounds decreased and led to the lower in vitro gas production (Kisworo et al., 2017). Previous study also reported that herbs (galangal) supplementation had positive effect in reducing in vitro gas production (Khattab et al., 2016). In contrary, Rahmy et al. (2019) demonstrated that supplementing 1-7% medicine herbs (Fennel, Melissa and Caraway) increased the in vitro gas production of diet, however, they did not mention the characteristics of plant secondary metabolites. The differences in results may be due to differences in nutrient composition of the diets and the biological activity of secondary metabolites in the herbal supplements. Jayanegara et al. (2012) reported that low concentrations of tannins highly affect the gas production and rumen fermentation product.
The treatment of BAF tends to produce low GPNSF value. This may be due to the decreased performance of non-soluble fraction degrading bacteria, such as cellulolytic bacteria. A. paniculata after flowering phase had high contents of tannins and condensed tannin (Figure 1), thus there may be inhibition of bacterial performance. Plant secondary metabolites inhibit rumen microbial growth, reducing the digestibility of roughage due to inhibitory effect on proteolytic and fibrolytic bacteria (Kisworo et al., 2017). Phenolic compounds had negative effect for many rumen microbes, especially ciliate protozoa, fibrolytic bacteria and methanogens (Rira et al., 2015). Supplementing 0.5% before and 0.5% A. paniculata after flowering phase actually tends to increase optimum gas production (a+b) and IVOMD. The mechanism of the combination of this two harvesting phase of A. paniculata leaves needs further investigation. Szulc et al. (2020) reported that herbal mixtures influenced the cellulolytic bacterial activities (F. succinogenes, R. albus and R. flavefaciens) and increased digestibility. The improvement of IVOMD and gas production after supplementation by herbal commercial products in feed may be due to enhanced microbial activity after supplementation (Jain et al., 2017). Regardless of the type of herbs, the interaction between supplements and nutrient compounds in the basal diet also needs to be considered in the application of mixed herbs supplementation. Previous studies using high nutrient rations showed increasing tendency towards IVOMD, gas production and rumen fermentation products (Jain et al., 2017). Variation in the results of in vitro fermentation characteristics may be due to several factors such as difference in nutrient component, sample processing and handling of research equipment (Rahmy et al., 2019).
Overall, supplementing A. paniculata leaves showed no significant effect on gas production and IVOMD. This is in agreement with Yusuf et al. (2012) who reported similar results for A. paniculata addition in diet. Herbal (thyme + celery) plant supplementation (2.5 to 10 g/kg DM) had no significant effect on ruminal gas production (El Tawab et al., 2021). Wadhwa et al. (2020) demonstrated that the net gas production of total mixed ratio (TMR) diets did not influenced by Acacia catechu, Acacia nilotica and Areca catechu herbal inclusion. Supplementing herbs (galangan and lemongrass) on basal diets had no effect on dry matter and organic matter digestibility (Khattab et al., 2016).
The pH is an effective indicator for fermentation condition in the rumen. In present study, the ruminal pH values ranged from 6.51-6.68 and it is ideal for optimum rumen fermentation conditions (Mcdonald et al., 2010). The results of the present study was in agreement with Karnani et al. (2021) and El Tawab (2021) where the supplementation of herbal product did not influence the ruminal pH. In contrast, supplementing A. paniculata leaves could increase ruminal pH due to increasing of Ruminococcus albus, Ruminococcus flavefaciens and Fibrobacter succinogenes population (Yusuf et al., 2017). This differences might be due to variation in nutrient composition of basal diet. Ammonia, as a major source of nitrogen, is very important for rumen microbial protein synthesis (Kisworo et al., 2017). In the present study, there was no significant difference observed in NH3 concentration. The present results are in agreement with Kisworo et al. (2017) who reported that insignificant NH3 concentration among treatments may be caused by the indifferent protein content between treatments (Kisworo et al., 2017). In contrast, Yusuf et al. (2014) reported that the supplementation of both leaf and whole plant of A. paniculata reduced the concentration of NH3 in Boer diets. Karnani et al. (2021) also observed that the concentration of NH3 in herbal product supplemented group decreased significantly in rumen liquor. The use of lower dose of herbal supplement may result in variable values on NH3 concentration.
Total volatile fatty acids showed no significantly difference between A. paniculata treatments compared with control group. These results are in agreement with Wadhwa et al. (2020) who observed that at low level of Areca catechu or Acacia nilotica (2%) did not reduced TVFA. El Tawab et al. (2021) also demonstrated that there are no significantly effect between mixed herbal inclusion compared with basal diet on TVFA values. Similar findings also noted by Yusuf et al. (2017) for A. paniculata supplementation, both in whole-plant and leaves application. This indicates that A. paniculata as herbal supplement has no negative effect on rumen fermentation. Using medical herbs in animal diets had no negative effect on major cellulolytic bacteria activity and ruminal fermentation (Naumann et al., 2018; El Tawab et al., 2021). The lower dose of herbal plants also influence the non-reactive effect in the ruminal ecosystem (Szulc et al., 2020).
CONCLUSION
Total tannins and condensed tannin contents of A. paniculata leaves were greater in plants after flowering than before flowering phase. However, there were no significant differences in biological activity of tannins between two harvesting times. The supplementation of A. paniculata leaves before and after flowering phase in the diet does not influence the digestibility. The implication of this study is that the combination of sambiloto before and after flowering phase can be used as an herbal supplement without reducing the rumen fermentation process. Further study to examine the effect of A. paniculata supplementation on the health of livestock is suggested.
ACKNOWLEDGEMENTS
The authors acknowledge financial support from Center for Isotopes and Radiation Application (CIRA), National Nuclear Energy Agency of Indonesia (BATAN). All authors are please to thank Mr. Udin Siman for laboratory assistance. We are thankful for the kind support from Science and Technology Scholarship, Ministry of Research and Technology/National Research and Innovation Agency of Indonesia.
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
AUTHORS CONTRIBUTION
Priyoatmojo designed the experiment, prepared raw sample, checked data and revised the article draft; Maharani performed the chemical composition analyses and in vitro digestibility assay; Ansori performed the sample preparation and in vitro digestibility assay; Hardani conducted the total phenol and tannin measurements; Trinugraha checked data analysis and revised the article draft; Handayani performed the in vitro digestibility assay and revised the manuscript; Sasongko prepared raw sample and measured phenolic compounds; Wahyono supervised the experiment, collected the data, wrote the first draft and revised the manuscript.
REFERENCES






