Advances in Animal and Veterinary Sciences
Research Article
Assessment of Phytochemical, Antioxidant and Antibacterial Activity of Balanites Aegyptiaca and Curcuma Longa against Some Bacterial Pathogens Isolated from Dairy Cow Infected with Mastitis
Doaa Sedky1*, Amany M. Mohamed1, Rasha Fouad2, Manal H.M. Khafagi1, Elsayed A. Omer2, Mohamed K. Elbayoumy1, Mohammad M. Effat3, Hala A. A. Abou-Zeina1
1Department of Parasitology and Animal Diseases, Veterinary Research Institute, National Research Centre, 33 Bohouth St., Dokki, 12622 Giza, Egypt; 2Department of Medicinal and Aromatic Plants Research, National Research Centre, 33 Bohouth St., Dokki, 12622 Giza, Egypt; 3Department of Microbiology and Immunology, Veterinary Research Institute, National Research Centre, 33 Bohouth St., Dokki, 12622 Giza, Egypt.
Abstract | Bovine mastitis is very important disease threatening the dairy industry and animal wealth globally, resulting in great economic losses. The present work was aimed to assess the phytochemical components, antioxidant activity and the antibacterial effectiveness of Balanites aegyptiaca fruits (BAF) and Curcuma longa powder (CLP) extracts. A total of 287 quarter milk samples were collected from dairy cows raised in four different governorates of Egypt; 95 from clinical mastitis, 37 from subclinical mastitis and 155 from normal milk samples. The isolates from milk samples were identified and differentiated by microbiological cultures, colony morphology, hemolytic activity of the colony, Gram’s stain and biochemical tests. Antibacterial activity of the aqueous extract of BAF (AE-BAF) and ethanolic extract of CLP (EE-CLP) at different concentrations were investigated against Gram-positive and Gram-negative bacteria isolated from clinical and subclinical mastitic cows using agar well diffusion technique. The isolated bacteria from collected milk samples were Staphylococcus aureus (30.30%), Escherichia coli (28.03%), Streptococcus agalactiae (19.70%), Salmonella spp. (10.60%), Bacillus subtilis (6.06%), Klebsiella pneumoniae (3.03%) and Pseudomonas aeruginosa (2.27%). Preliminary phytochemical screening of AE-BAF indicated the presence of flavonoids, saponins, tannins, phenols, carbohydrate, cardiac glycoside, terpenoids and steroids. Except the cardiac glycoside, the EE-CLP contains the same components. The antioxidant efficacy of the tested plant extracts was evaluated by using free radical scavenging assay method. The AE-BAF and EE-CLP posses 86.6% and 85.9% free radical scavenging activity with 1.5 and 0.125 mg /ml concentrations, respectively. The AE-BAF possess significant antibacterial activity at 400 and 800 mg/ml against all isolates, except K. pneumoniae which was not suppressed at the 400 mg/ml concentration. The significant (P ≤ 0.05) antibacterial activities of EE-CLP were observed at 20 and 50 mg/ml concentrations. It could be concluded that AE-BAF and EE-CLP exhibited potent in-vitro antibacterial activities, thus justifying their application in treatment of clinical cases of bovine mastitis.
Keywords | Bovine mastitis, Balanites aegyptiaca, Curcuma longa, Phytochemical, Antioxidant and antibacterial
Received | September 22, 2021; Accepted | October 10, 2021; Published | December 01, 2021
*Correspondence | Doaa Sedky, Department of Parasitology and Animal Diseases, Veterinary Research Institute, National Research Centre, 33 Bohouth Street, Dokki, P.O. Box 12622, Giza, Egypt; Email: [email protected]
Citation | Sedky D, Mohamed AM, Fouad R, Khafagi MHM, Omer EA, Elbayoumy MK, Effat MM, Abou-Zeina HAA (2022). Assessment of phytochemical, antioxidant and antibacterial activity of balanites aegyptiaca and curcuma longa against some bacterial pathogens isolated from dairy cow infected with mastitis. Adv. Anim. Vet. Sci. 10(1): 160-169.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.1.160.169
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2022 Sedky et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Bovine mastitis is the most common and commercially significant infectious disease in the world. Bovine mastitis, which affected both milk output and milk quality, resulted in increased treatment expenses, labour expenditures, and culling, resulting in significant economic losses (Kotb et al., 2021). Mastitis caused due to a variety of agents including environmental and microbial factors (Pyörälä, 2002). Mastitis in cattle is produced by a variety of pathogenic microorganisms. Staphylococcus aureus (S. aureus), Streptococcus agalactiae (S. agalactiae), Streptococcus uberis (S. uberis), Streptococcus dysgalactiae (S. dysgalactiae) and Escherichia coli (E. coli) are the most common pathogens accounting for the majority of cases being (Reyher et al., 2012). The distribution of mastitis pathogen strains differs within individual animals in a herd, as well as herds, host species and countries (Sedky et al., 2020a). The frequent use of antibiotics intra-mammary and parenterally in the treatment of mastitis has several adverse effects, including antibiotic residues entering the human food chain and the transmission of antibiotic-resistant bacteria strains (Krömker and Leimbach, 2017; Mohandes et al., 2021). Additionally, antibiotic-resistant resulting in low rate of intramammary cures (Dingwell et al., 2003).
Controlling udder health is essential for the dairy production and reducing food borne illness and giving healthy dairy food (Fusco et al., 2020). Furthermore, the development and spread of antibiotics resistance bacteria as a result of mastitis treatment is a public health hazard to consumers, affecting both human and animal health (Oliver et al., 2005). There is a considerable interest in developing new antimicrobial drugs from medicinal plants because of increasing evidence of multidrug-resistant bacteria (Kovacevic et al., 2021).
Balanites aegyptiaca Delile (L.) (Family: Balanitaceae) is commonly known as the desert date, is a spiny tree that grows up to 10 meters tall and is found in Africa and South Asia. B. aegyptiaca grows in Western and Eastern deserts of Egypt and at the borders between Egypt and Sudan (Abdel-Farid and El-Sayed, 2021). B. aegyptiaca fruit is reported to have a wide variety of compounds with different biological and pharmacological effects such as anti-inflammatory, antioxidant, cytotoxic and antimicrobial activities (Chothani and Vaghasiya, 2011).
Desert date is rich in antimicrobial compounds including phenolics as simple phenols, quinones, phenolic acids, flavonols, flavones, flavonoids, tannins, coumarins, terpenoids, essential oils and alkaloids (Cowan,1999; Murthy et al., 2021). In African folk medicine the bark of B. aegyptiaca is commonly used for the treatment of wounds and skin diseases. The aqueous extract of B. aegyptiaca bark inhibited the growth of Pseudomonas aeruginosa (P. aeruginosa) and S. aureus isolated from wounds (Anani et al., 2015). The antifungal activity of saponin-rich extracts of fruit mesocarp against phytopathogenic fungi was investigated in vitro (Chapagain et al., 2007).
Curcuma longa L. (Turmeric) belongs to the Zingiberaceae family botanically. Turmeric has antibacterial, antifungal, antiviral, anti-aging, antimalarial, anticancer, anti-Alzheimer’s disease, antioxidant, and anti-inflammatory properties (Moghadamtousi et al., 2014; Boroumand et al., 2018). The curcumin and the oil fraction inhibit the growth of many bacteria such as E. coli, Streptococcus, Salmonella Typhimurium, Staphylococcus, Yersinia enterocolitica, Bacillus subtilis and B. cereus (KaiKai et al., 2020).
The main objective of this study was to evaluate the prevalence of mastitis in dairy cattle and the principal bacterial pathogens that cause mastitis in four Egyptian governorates, and assess the antibacterial activities of B. aegyptiaca fruits and Curcuma longa powder as a model of medicinal plants against mastitis-associated pathogens. The purpose was also to assess the phytochemical contents and the in vitro antioxidant capabilities of the medicinal plant extracts studied.
MATERIALS AND METHODS
Ethical approval
The Ethical Committee for Medical Research (ECMR) at the National Research Centre (NRC) in Egypt approved this study and in accordance with local laws and regulations. Approval Protocol No.: 16229.
Collection of milk samples
After clinical examination of udder, milk samples were aseptically collected as previously described by Amer et al. (2018). California mastitis test (CMT) were applied for detection of clinical and subclinical mastitis (Sedky et al., 2020b). The quarter which gave positive result for CMT was considered as sub-clinically affected.
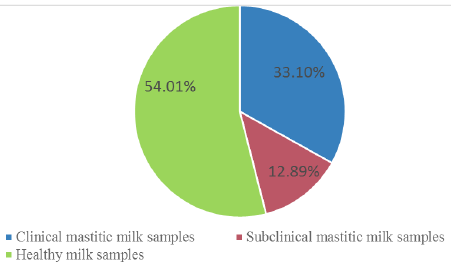
A total of 287 milk samples (95 from clinically mastitis, 37 from subclinical mastitis and 155 normal milk samples) were obtained from cows housed in private dairy farms located in four different provinces of Egypt at Kaluobia- Giza –Sharkia and EL Fayoum, 87, 95, 70 and 35 milk samples were collected, respectively.The distribution of the collected milk samples were illustrated in Table 1.
Bacteria isolation and identification from milk samples
Microbiological protocols for the diagnosis of udder infection published by the National Mastitis Council (1999), were used to isolate and identify bacterial strains from milk samples. A loopful of the milk sample was streaked on blood agar and then sub cultured on Eosin methylene blue (EMB), Mannitol salt agar, Edwards Agar, Salmonella-Shigella Agar, and MacConkey Agar. After that, plates were then incubated aerobically at 37ºC for 24 hours. Morphology of the suspected colonies were examined microscopically in Gram stained films. They were then subjected for biochemical tests for identification of pathogens including oxidase activity, acid production (lactose sucrose and glucose fermentation), indole production, catalase test, mannitol fermentation test, coagulase test, Voges–Proskauer and hydrogen sulfide production as formerly designated by Quinn (2011). In addition, each strain was confirmed the identification by using Analytical Profile Index API-20 tests (API, bio Meraux, France).
Table 1: Incidence of clinical, subclinical mastitic and healthy milk samples in four different provinces.
| Provinces |
clinical mastitic milk samples |
Subclinical mastitic milk samples |
Healthy milk samples |
Total milk samples |
| Kaluobia | 23 (26.44%) | 21 (24.14%) | 43 (49.43%) | 87 |
| Giza | 31 (32.63%) | 6 (6.32%) | 58 (61.05%) | 95 |
| Sharkia | 27 (38.57%) | 8 (11.43%) | 35 (50.00%) | 70 |
|
El-Fayoum |
14 (40%) | 2 (5.71%) | 19 (54.29%) | 35 |
| total | 95 (33.10%) | 37 (12.89%) | 155(54.01%) | 287 |
Preparation of plant extracts
Ethanolic extract of C. longa
C. longa dried rhizomes were purchased from a local market in Cairo, Egypt. It was extracted according to the techniques described by El-Newary et al. (2016). Briefly, dried rhizomes were coarsely powdered, then C. longa powder (1kg) were soaked in 4 L. 70% ethyl alcohol, and kept in tightly sealed vessels at room temperature for three weeks, stirring several times daily with a sterile glass rod. This mixture was filtered. The extraction of the residue was repeated 3-5 times in the same manner until a clear colorless supernatant extraction liquid was obtained. The extracted liquid was filtered and concentrated using rotary evaporator under reduced pressure at 50oC until the solvent was completely removed. The extract was stored at 4°C until used.
Aqueous extract of B. aegyptiaca
The fruits of B. aegyptiaca were obtained from Aswan, Egypt. The fresh fruits of B. aegyptiaca (1kg) were subjected to triple maceration then they were soaked in 4L distilled water and continued the next steps as shown previously in the EE-CLP.
Phytochemical screening
The AE-BAF and EE-CLP were analyzed for the presence of the phytoconstituents through preliminary phytochemical screening using modified and standard methods (Obasi et al., 2010; Tiwari et al., 2011). The plant extracts were tested for the following phytochemical components: phenolics, cardiac glycosides, tannins, saponins, alkaloids, steroids, flavonoids, carbohydrates, proteins and terpenoids.
In vitro antioxidant activity
The antioxidant activities of AE-BAF and EE-CLP were assessed in terms of hydrogen-donating or radical-scavenging ability, using the stable radical DPPH (2, 20-Diphenyl-1-picrylhydrazyl) according to Brand-Williams et al. (1995). This activity is comparable with that of the standard drug ascorbic acid as strong antioxidant. The DPPH solution was prepared by dissolving 9.85 mg in 100 ml 70% methanol. Different concentrations of AE-BAF and EE-CLP were added to methanol 70 % and 335 µl DPPH. The mixtures were thoroughly shaken before being placed in the dark at room temperature for 30 minutes. If free radicals have been scavenged the purple colour of DPPH will degenerate to yellow, indicating free radical scavenging activity. A spectrophotometer was used to measure the decrease in absorbance at 517 nm. The Absorbance of the radical without sample was used as control (665 µl methanol 70 % and 335 µl DPPH). The amount of sample required to reduce the DPPH absorbance was determined.
The inhibition percentage of the DPPH radical by the extracts was calculated according to the following equation:
DPPH inhibition % = [(AB - AS)/AB] x 100
Where, AB=absorbance of control sample, and AS= absorbance of a tested sample at the end of the reaction.
Assay of the antibacterial effect of the plant extracts
Inoculums preparation
The identified isolated micro-organisms were cultured on a nutrient agar plate and incubated at 37°C for 24 hours. Discrete colonies were collected with sterile wire loop and inoculated into a 5ml sterile saline solution. To obtain 108 CFU/ml bacterial cultures, the test tubes were thoroughly shaken and the turbidity of the bacterial suspension was adjusted by comparing it with the 0.5 McFarland standards using spectrophotometer. Its absorbance was set to 580 um (Mostafa et al., 2018).
Agar well diffusion test
Muller Hinton agar was poured into sterile Petri dishes and was allowed to solidify. Using a sterile swap stick one milliliter of fresh bacterial culture was spread on the surface of the media. A sterile cork borer 6 mm in diameter was used to make wells in agar plates containing inoculums. Then, 100 μl of different concentrations of EE-CLP100, 50, 20 and 10 mg/ml then dispensed into the wells. The plates were placed in the refrigerator for 30 minutes to allow the extracts to full then diffuse into the agar. The plates were then incubated at 37°C for 18 hours. The zone of inhibition that appeared after the incubation period was recorded to detect antimicrobial activity (Ahuja et al., 2015). Gentamycin was used as positive control as described by NCCL (1999). The experiment was carried out in triplicate, and the results were expressed as means ± standard error of the three parallel measurements. This procedure was repeated again by using 800 and 400mg/ml of AE-BAF. Different concentrations of the extracts were prepared separately by dissolving the extract in sterile distilled water.
Statistical analysis
Using SPSS (version 17), data were statistically analyzed according to Snedecor and Cochran (1982). Data are represented as mean and standard error (mean ± SE). Using one-way analysis of variance (ANOVA) and Duncan’s multiple range test, differences between zones of inhibition (mm) induced by different concentrations of tested plant extracts and the antibiotic against bacteria, as well as between means in different bacteria species, were tested for significance. At the P ≤ 0.05 level of probability, differences were judged significant.
RESULTS
Bacteria isolation and identification from milk samples
The incidence of clinical, subclinical mastitis and healthy collected milk samples in all provinces is demonstrated in Figure 1 and Table 1. Out of 287 cow’s milk samples 132 isolates were identified and differentiated from 95 clinical and 37 subclinical masitic milk samples. Bacterial isolates were S. aureus (30.30%), E. coli (28.03%), S. agalactiae (19.70%), Salmonella species (10.60%), Bacillus subtilis (B. subtilis) (6.06%), Klebsiella pneumoniae (K. pneumoniae) (3.03%) and Pseudomonas aeruginosa (P. aeruginosa) (2.27%) as shown in Table 2.
Phytochemical screening
The results of preliminary phytochemical analysis of the different crude extracts of the medicinal plants in respect; AE-BAFand EE-CLP are presented in Table 3. The results show that all of the phytochemical compounds that were tested were present in the in AE-BAF, while EE-CLP contains all the same components, with the exception of cardiac glycosides which were not present.
In vitro antioxidant effect
The obtained results indicated that the extracts of tested medicinal plants showed scavenging activity and illustrated in Figure 2A, B, C. It is seen that the AE-BAF possess high antioxidant activity at higher concentration (86.6% with 1.5mg/ml), whereas the EE-CLP possess good antioxidant activity (85.9% with 0.125mg/ml). The ascorbic acid possesses antioxidant activity at very high concentration where the percentage of scavenging activity was 92.73 % with 50mg/ml concentration.
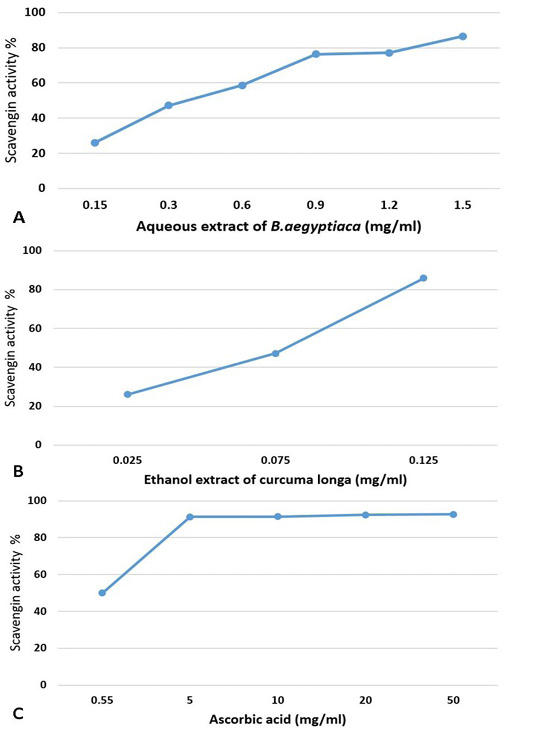
Figure 2: Antioxidant activity of different concentrations of aqueous extract of B. aegyptiaca (A); ethanol extract of Curcuma longa (B) and ascorbic acid (C) using DPPH method.
Table 2: Percentage of isolated bacteria from clinical and subclinical mastitic cow’s milk in four provinces.
| Provinces | Total CM and SCM |
Isolated bacteria (n (%)) |
||||||
|
Gram -ve bacteria |
Gram + ve bacteria |
|||||||
| E.coli | K. pneumoniae | P. aeruginosa |
Salmonella spp. |
S. aureus | S. agalactiae | B. subtilis | ||
| Kaluobia | 44 | 14(31.81%) | 2(4.56%) | 1(2.27%) | 7(15.90%) | 9(20.45%) | 7(15.90%) | 4(9.09%) |
| Giza | 37 | 10(27.03%) | 0(0%) | 2(5.41%) | 3(8.11%) | 16(43.24%) | 5(13.51%) | 1(2.70%) |
| Sharkia | 35 | 8(22.86%) | 2(5.71%) | 0(0%) | 2(5.71%) | 8(22.86%) | 12(34.29%) | 3(8.57%) |
| El-Fayoum | 16 | 5(31.25%) | 0(0%) | 0(0%) | 2(12.50%) | 7(43.75%) | 2(12.50%) | 0(0%) |
| Total | 132 | 37(28.03%) | 4(3.03%) | 3(2.27%) | 14(10.60%) | 40(30.30%) | 26 (19.70%) | 8(6.06%) |
CM and SCM= clinical mastitic and subclinical mastitic milk samples.
Table 3: Phytochemical constituents of different crude extracts of B. aegyptiaca fruits and C. longa powder.
| Test |
Aqueous extract of B. aegyptiaca |
Ethanol extract of C. longa |
| Phenolics | + | + |
| Alkaloids | + | + |
| Steroids | + | + |
| Cardiac Glycosides | + | - |
| Tannins | + | + |
| Saponins | + | + |
| Flavonoids | + | + |
| Proteins | + | + |
| Carbohydrates | + | + |
| Terpenoids | + | + |
( + )= Detected, (-) = Not detected
Antibacterial activity of plant extracts
Evaluation of the antibacterial activity of AE-BAF against the bacteria isolated from clinical and subclinical mastitic cow’s milk was recorded in Table 4 and illustrated in Figure 3. The results revealed that AE-BAF at 400 and 800 mg/ml had the potential to limit growth of all bacteria except K. pneumoniae, which was not suppressed at the 400 mg/ml concentration. When AE-BAF was compared with the control antibiotic (Gentamycin), it showed considerable (P ≤ 0.05) antimicrobial activity against P. aeruginosa, Salmonella species and S. aureus at 800 mg/ml concentration. Antibacterial activity of AE-BAF at 400 mg/ml concentration was found to be substantial (P ≤ 0.05) against P. aeruginosa and S. aureus when compared to the Gentamycin. There were no significant differences in antibacterial activity between the two concentrations of AE-BAF against all bacteria, with the exception of K. pneumoniae, Salmonella species and S. aures which were considerably (P ≤ 0.05) suppressed at the 800 mg/ml concentration. Among bacteria species, S. agalactiae and P. aeruginosa were the most significantly (P ≤ 0.05) inhibited bacterium species at 400 mg/ml, while S. agalactiae was the most inhibited bacteria species at 800 mg/ml, compared to other species.
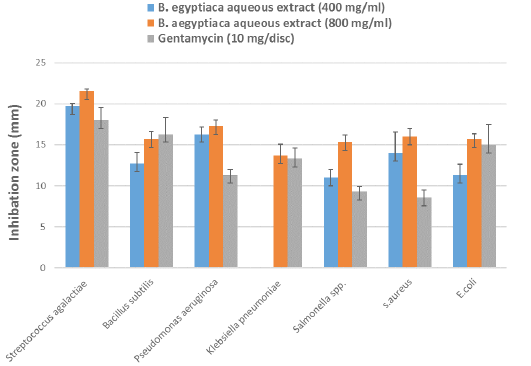
Figure 3: Antibacterial effect by different concentrations of B. aegyptiaca aqueous extract and Gentamycin antibiotic against bacteria isolated from clinical and subclinical mastitic cow’s milk.
The antimicrobial properties of the EE-CLP against bacteria isolated from clinical and subclinical mastitic cow’s milk presented in Table 5 and Figure 4, where all the test organisms were inhibited with different concentrations of extract. The significant (P ≤ 0.05) antibacterial activity of EE-CLP was observed at 20 and 50 mg/ml concentrations against S. aureus compared to the Gentamycin. There were no significant differences in antimicrobial activity between various concentrations of EE-CLP and the control antibiotic against S. agalactiae, P. aeruginosa, K. pneumoniae, Salmonella species, and E. coli, except that 100mg/ml which had a significantly (P ≤ 0.05) lower effect against B. subtilis compared to concentration 20, 50 and the antibiotic. While concentration 50 had a significant (P ≤ 0.05) higher effect against S. aureus compared to the Gentamycin and, 10 and 100 mg/ml. At concentrations of 10, 20 and 100 mg/ml, S. agalactiae is the most affected (P ≤ 0.05) among bacterium species, whereas there was no significant difference between them at concentration 50mg/ml.
DIscussion
Mastitis is considered the most common serious health problem on dairy farms which lead to significant bio health hazard to human particularly in developing countries such as in Egypt (Ammar et al., 2016). Clinical inspection and the California mastitis test were found to be effective diagnostic tools for detecting and distinguishing clinical and subclinical mastitis from apparently healthy cattle, which is consistent with previous findings (Gianneechini et al., 2002).
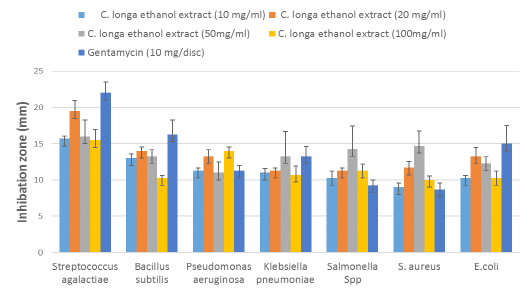
Figure 4: Antibacterial activity of the different concentrations of C. longa ethanol extract against bacteria strains isolated from clinical and subclinical mastitic cow’s milk compared with antibiotic.
According to clinical inspection and CMT obtained results, the prevalence of clinical and subclinical mastitic and normal milk samples in total collected samples were 33.10%, 12.89% and 54.01 %, respectively. These findings were nearly similar to those reported by (Zeedan et al., 2014), who recorded 34.5%, 24.7%, 40.8% prevalence of clinical mastitis, subclinical mastitis and healthy dairy cows respectively, at Beni-Suef, El-Fayoum, Behera and Monofia Governorates in Egypt. However, they disagree with (Sayed et al., 2014), who reported that 56.3% of mastitis was subclinical and 13.3% was symptomatic. This variation in the percentage of clinical and subclinical mastitis could be attributed to differences in hygienic condition and management in rural areas (Zeedan et al., 2014).
Bacteria are the most important microorganisms that cause mastitis in dairy herds, and they can act as a contagious pathogen or an opportunistic pathogen (Tarazona-Manrique et al., 2019). A total of 132 different bacterial isolates were isolated from 287 cow’s milk samples; they were identified by biochemical tests and found to belong to seven different species, as shown in Table 2. Das et al. (2010) and Alkhamaiseh et al. (2011) believe that bacterial culture is a good standard method for isolating causative bacteria.
The most predominant bacteria identified in milk samples from clinical mastitic and subclinical cows were S. aureus (30.30%), E. coli (28.03%) and S. agalactiae (19.70%). The highest prevalent of S. aureus, E. coli and S. agalactiae may occur through transmission by the contaminated Milker’s hands, teat-to-teat hygiene and cow-to-cow. These findings were in line with Das et al. (2010), who considered these microorganisms are the major etiological agents of clinical and subclinical mastitis worldwide. Because E. coli infects the udder via the teat canal, a higher incidence rate of E. coli could be attributed to poor hygienic conditions of the environment. S. aureus causes food poisoning and commonly found in milk from dairy animals that have mastitis from the environment (Mir et al., 2014).
Other species of bacterial isolates in this study, as Salmonella species, B. subtilis, K. pneumoniae and P. aeruginosa are minor causes of bovine mastitis, and this is in agreement with (Sayed et al., 2014), who isolated also Salmonella species in low incidence (4.2%), K. pneumoniae (8.5%), and P. aerguinosa (4.2%), as well as Salih and Ahmed (2011), who isolated B. subtilis (9%) from cow’s milk. The distribution of mastitis pathogen strains differs within individual animals in a herd, among herds and among host species (Sedky et al., 2020a).
In the present results, phytochemical screening of the AE-BAF detected the presence of alkaloids, flavonoids, steroids, saponins, phenolic compounds, terpenoids and tannins. This was consistent with the work of Abdulhamid and Sani (2016), who detected the presence of the same phytochemicals in the AE-BAF. It was slightly similar to the observations of Henna et al. (2010), who reported the presence of the same phytochemicals in the AE-BAF except steroids and alkaloids were not detected in their study. This is in line with other phytochemical study of Wakawa et al. (2018), who demonstrated the same in the AE-BAF, except cardiac glycosides and alkaloids were not detected in their study.
Phytochemical screening of the EE-CLP indicated the presence of flavonoids, steroids, saponins, alkaloids, phenolic compounds, terpenoids and tannins, these findings are in agreement with Oghenejobo et al. (2017), who detected the same compounds in the EE-CLP. Also phytochemical screening of the C. longa powder confirmed the presence of carbohydrates, proteins and amino-acids, alkaloids, terpenoids and flavinoids but no tannins, glycosides and saponins (Pawar et al., 2014). Contrary to the findings of Pawar et al. (2014), tannins and saponins were found in EE-CLP in this study. The chemical composition, toxicity, and bioactivity of the extracts may differ due to the solubility of the active component in various solvents (Ekpo and Etim, 2009), the chronological age of the plant, percentage humidity of the harvested material, and time of harvest, as well as the method of extraction (Felix, 1982).
The active phytochemical constituents indicated in these results as shown in Table 3 alone or in combination may be responsible for the observed antioxidant activity (Paloi and Acharya, 2014). Plant phenols, flavonoids, tannins and terpenoids are reported to possess potent antioxidant activity (Maryam et al., 2009; Khatua et al., 2013). Usman et al. (2020); Chothani and Vaghasiya (2011) reported that different parts of B. egyptiaca possess potent antioxidant activity. Miquel et al. (2002) reported that curcumin exhibit more potent antioxidant activity comparable of that to vitamin E.
The AE-BAF possess significant antibacterial activity at 400 and 800 mg/ml against the Gram-positive and Gram-negative bacteria isolated from clinical and subclinical mastitic cow as shown in Table 4 and Figure 3. The results obtained showed that the antibacterial activity of the extract significantly increased with increasing concentration, which is consistent with the findings (Ezemokwe et al., 2020). Furthermore, these results were supported by Jahan et al. (2013), who found that B. aegyptiaca have a broad spectrum activity against various bacteria including S. aureus, S. pyogenes, E. coli, K. pneumoniae, P. aeruginosa, S. typhimurium, B. subtilis and resistance bacteria harboring bla genes. Also, Ibrahim (2016) reported that AE-BAF was more potent than petroleum ether extract of Phyllanthus reticulatus against S. aureus and E. coli. It has been reported that B. aegyptiaca extracts inhibited the in vitro growth of several strains of S. aureus and P. aeruginosa (Anani et al., 2015; Mutwali and Abdelgadir, 2016).
The presence of saponins and alkaloids in this plant may be responsible for its antibacterial effect, as some previous research have suggested (Doughari et al., 2007). The presence of the phytochemical constituents in B. aegyptiaca extracts in this study was reported to be responsible for the plant extract’s antibacterial action (Yadav and Panghal, 2010; Henna et al., 2010).
Table 4: Mean zones of inhibition (mm) ± standard error by different concentrations of B. aegyptiaca aqueous extract and antibiotic against bacteria isolated from clinical and subclinical mastitic cow’s milk.
| Test pathogens | B.aegyptiaca aqueous extract (400 mg/ml) | B. aegyptiaca aqueous extract (800 mg/ml) |
Gentamycin (10 mg/disc) |
F value | P value |
| Streptococcus agalactiae |
19.7±0.33Aa |
21.5±0.29Aa |
18.0±1.52Aa |
3.637 | NS |
| Bacillus subtilis |
12.7±1.33BCa |
15.7±0.89BCa |
16.3±2.02Aa |
1.717 | NS |
| Pseudomonas aeruginosa |
16.3±0.88ABa |
17.3±0.67Ba |
11.3±0.66BCb |
18.600 | 0.003 |
| Klebsiella pneumoniae |
0.0±0.00Db |
13.7±1.33Ca |
13.3±1.33ABCa |
51.281 | <0.001 |
|
Salmonella Spp. |
11.0±1.00Cb |
15.3±0.88BCa |
9.3±0.67Cb |
12.950 | 0.007 |
|
S. aureus |
14.0±2.52BCab |
16.0±1.00BCa |
8.6±0.89Cb |
5.315 | 0.047 |
|
E. coli |
11.3±1.33Ca |
15.7±0.66BCa |
15.0±2.51ABa |
1.909 | NS |
| F value | 22.516 | 8.072 | 5.400 | ||
| P value | <0.001 | 0.001 | 0.004 |
Different small letters at the same row indicate to significant differences between different concentrations of B. aegyptiaca aqueous extract and antibiotic, while different capital letters at the same column indicate to significant differences between bacteria species at P ≤ 0.05, NS= Non-significant.
Table 5: Mean zones of inhibition (mm) ± standard error by different concentrations of C. longa ethanol extract and antibiotic against bacteria isolated from clinical and subclinical mastitic cow’s milk.
|
Test pathogens
|
C. longa ethanol extract (10 mg/ml) |
C. longa ethanol extract (20 mg/ml) |
C. longa ethanol extract (50mg/ml) |
C. longa ethanol extract (100mg/ml) |
Gentamycin (10 mg/disc) | F value | P value | |
| Streptococcus agalactiae |
15.7±0.33Aa |
19.5±1.44Aa |
16±2.30Aa |
15.5±1.44Aa |
22±1.52Aa |
1.283 | NS | |
| Bacillus subtilis |
13.0 ±0.57Bab |
14.0±0.58Ba |
13.3±0.88Aab |
10.3±0.33Cb |
16.3±2.02Aa |
4.088 | 0.032 | |
| Pseudomonas aeruginosa |
11.3±0.33Ca |
13.3±0.88Ba |
11.0±1.52Aa |
14.0±0.57ABa |
11.3±0.66BCa |
2.333 | NS | |
| Klebsiella pneumoniae |
11.0±0.58Ca |
11.3±0.33Ba |
13.3±3.38Aa |
10.7±1.20Ca |
13.3±1.33ABCa |
0.559 | NS | |
| Salmonella Spp. |
10.3±0.88CDa |
11.3±0.33Ba |
14.3±3.17Aa |
11.3±0.88BCa |
9.3±0.66Ca |
1.432 | NS | |
| S.aureus |
9.0±0.58Db |
11.7±0.88Bab |
14.7±2.02Aa |
10.0±0.57Cb |
8.7±0.88Cb |
4.763 | 0.021 | |
| E.coli |
10.3±0.33CDa |
13.3±1.20Ba |
12.3±0.88Aa |
10.3±0.88Ca |
15±2.51ABa |
2.129 | NS | |
| F value | 15.947 | 10.149 | 0.533 | 5.483 | 5.400 | |||
| P value | <0.001 | <0.001 | NS | 0.004 | 0.004 |
Different small letters at the same row indicate to significant differences between different concentrations of C. longa ethanol extract and antibiotic, while different capital letters at the same column indicate to significant differences between bacteria isolates at P ≤ 0.05, NS= Non significant.
This study evaluated the antibacterial effects of the crude EE-CLP, ethanolic extract of C. longa inhibits the growth of both Gram-positive and Gram-negative bacteria as shown in Table 5 and Figure 4. The significant antibacterial activities were observed with 20 and 50 mg/ml concentrations. These results are in agreement with those of Jha et al. (2013), who investigated the effects of turmeric and curcuminoids against S. aureus, B. subtilis and P. aeruginosa bacteria. They mentioned that the highest antimicrobial activity resulted from ethanol extract at 20 mg/ml. The C. longa rhizome extract was founded to be more potent antibacterial agent than the leaf extract (Singh et al., 2017), and when ethanol was utilized as the extraction solvent, higher quantities of curcumin and antimicrobial activity were produced (Martinez-Correa et al., 2017).
The antibacterial activity of ethanolic extract was due to the presence of various types of terpenoids and glycosides like compounds and these compounds have been found to have strong antimicrobial activity against Gram positive and Gram negative bacteria (Chhetri et al., 2008; Okigbo et al., 2009). Curcuma aromatica extract (Wild turmeric) was reported to have antibacterial activity against B. lichaniformis, Micrococcus leutus, and S. Thyphorium (Ara et al., 2011). They also discovered that the antimicrobial action is due to the presence of high phenolic content. According to Wilson et al. (2005), B.subtilis was the most sensitive organism to C. longa extract curcuminoid and oil extracts.
CONCLUSIONS and Recommendations
The prevalence of subclinical and clinical mastitis was measured in different Egyptian governorates, and the most common bacteria causing mastitis was identified. According to this study, the AE-BAF and EE-CLP can be used as an alternative to standard mastitis therapy because of their chemical composition, antioxidant capability, and efficiency against mastitis-associated bacteria. AE-BAF exhibited potent antibacterial activities at 800 mg/ml and EE-CLP exhibited potent antibacterial at 20 and 50 mg/ml against the Gram-positive and Gram-negative bacteria isolated from clinical and subclinical mastitic cow. However, more research into their applicability to mastitis-affected dairy cows is needed and further studies are needed to discover the definite fractions from C. longa and or B. aegyptiaca to explain the definite mechanism of action of the tested extracts as antibacterial.
ACKNOWLEDGMENTS
This work was financially supported by National Research Centre as a part of the project No.11020303-the 11th Research plan, under supervision of Prof. Dr. Hala A.A. Abou- Zeina
Novelty Statement
The AE-BAF and EE-CLP exhibited potent antibacterial activities against the Gram -positive and Gram-negative bacteria isolated from clinical and subclinical mastitic cow, thus justifying their application in treatment of clinical cases of bovine mastitis.
AUTHOR’S CONTRIBUTION
All authors shared equally in designing, conducting the study and writing the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES