Advances in Animal and Veterinary Sciences
Short Communication
Cytokine Pattern in Piglets in the Early Postnatal Period
Aleksey Shakhov1, Larisa Sashnina1, Mariya Adodina1, Evgeniy Mikhaylov1, Mariya Zheynes1, Kristina Kopytina1, Mikhail Syromyatnikov1,2,3*
1Federal State Budgetary Scientific Institution, All-Russian Veterinary Research Institute of Pathology, Pharmacology and Therapy, Voronezh, 394087, Russian Federation; 2Voronezh State University, Voronezh, 394018, Russian Federation; 3Voronezh State University of Engineering Technology, Voronezh, 394000, Russian Federation.
Abstract | The content of interleukins (IL-1β, IL-2, IL-4, IL-10), tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN -γ) in piglets blood serum in the early postnatal period was determined by the enzyme immunoassay (EIAs). In the colostrum of sows and the blood of pigs that did not suckle colostrum, the expression of the genes IL-1α, IL-10, actin proteins (reference gene) were determinate by real-time quantitative polymerase chain reaction (qPCR). The relative level of expression of the studied genes was carried out using 2 -∆∆Ct method. A high content of IL-1β, IL-2, IL-4, IL-10, TNF-α and IFN-γ cytokines was detected in colostrum of sows after farrow. It decreased in twenty-four hours and the levels of IL-1β and IL-10 with slight fluctuations remained in milk during the entire period of lactation, and IL-2 and TNF-α – up to 22 days, followed by a decrease in the amount of IL-2 and an increase in the content of TNF-α on day 26, respectively. The level of IL-4 and IFN-γ in comparison with the daily parameters increased on days 7 and 14 with a decrease in their content at the end of lactation in sows. No expression of IL-1α, IL-10 and actin cytokine genes was detected in colostrum either due to the presence of a large amount of fat in it or the absence of living cells from which sufficient mRNA could be isolated. The expression of IL-1α cytokine gene and the presence of IL-1β, IL-2, IL-4, IL-10, TNF-α and IFN-γ were recorded in the newborns before obtaining colostrum, indicating their synthesis by fetal cells, the number of which increased in piglets at the age of twenty-four hours due to their intake with colostrum. The daily parameters of IL-1β, IL-10 and TNF-α with small fluctuations are maintained in piglets throughout the entire suckling period. The content of IL-2 in them during the first week of life and IL-4 at the age of twenty-four hours significantly increases with a subsequent decrease to days 14 and 22, respectively, and by the end of the suckling period of growth. The level of IFN-γ in piglets increases during the entire period of suckling, despite a significant decrease in its amount in milk, at the end of lactation of sows, which indicates the synthesis of cytokine by cells of their own immune system. Registration of the IL-1α gene and the presence of cytokines IL-1β, IL-2, IL-4, IL-10, TNF-α and IFN-γ in the blood serum of piglets before colostrum intake indicate their synthesis by fetal cells. The data obtained are recommended for use in assessing the immune status of healthy piglets and piglets with pathology, completeness of recovery of animals, prophylactic and therapeutic efficacy of immunomodulating drugs.
Keywords | Blood serum, Cytokine gene expression, Interleukin, Milk, Piglets
Received | November 15, 2020; Accepted | January 20, 2021; Published | December 01, 2021
*Correspondence | Mikhail Syromyatnikov, Voronezh State University, Voronezh-394018, Russian Federation; Email: syromyatnikov@bio.vsu.ru
Citation | Shakhov A, Sashnina L, Adodina M, Mikhaylov E, Zheynes M, Kopytina K, Syromyatnikov M (2022). Cytokine pattern in piglets in the early postnatal period. Adv. Anim. Vet. Sci. 10(1): 120-125.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.1.120.125
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2022 Shakhov et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Numerous studies of recent years have established the role of cytokines, which are the main mediators of the immune system, in performing many physiological functions, participating in the formation and regulation of the body’s defense reactions against pathogens (Kogut, 2000; Arango Duque and Descoteaux, 2014; Teijaro, 2017; Kany et al., 2019; Mahmoud et al., 2020; Neamat-Allah et al., 2020, 2021). They play a key role in the regulation of innate immunity (Stenger and Röllinghoff, 2001; Lin and Karin, 2007; Striz et al., 2014; Mahmoud et al., 2020; Neamat-Allah et al., 2020) and the adaptive cellular and humoral immune response (Sivakumar et al., 2004; Neamat-Allah and Mahmoud, 2019; Neamat-Allah et al., 2019a, b).
To assess the state of the cellular components of the immune system, along with determining the quantitative content of immune cells belonging to different functional classes (T-lymphocytes and their subpopulations, B-lymphocytes, NK cells and others (Lutskiy et al., 2015), it is necessary to study the level of cytokines secreted by immunocompetent and infected cells or precursors of leukocytes formed in the bone marrow and regulating the type of immune response (Groot et al., 2005). The latter have not been studied well in piglets, and a few data available from scientific literature are contradictory.
A number of studies (Nguyen et al., 2007; Hlavova et al., 2011; Elahi et al., 2017) exclude the presence of IL-6, TNF-α, IL-10, IL-4, IFN-γ, which are transmitted exclusively through colostrum and milk, in the blood serum of the newborn piglets. However, Splichal et al. (1994) believe that already on the 26th day of gestation of sows, IFN-α secreting cells (SK) can be detected in the fetal liver, and the later stages of pregnancy can also be found in the cord blood, spleen and bone marrow, that is IFN-α SK appears very early and may represent an early mechanism of antiviral defense.
There are reports on expression of TNF-α by lymphocytes and / or macrophages of the pig fetus after the 37th day of gestation, and interferon by macrophage and not lymphoid cells (Trebichavsky et al., 1995). Malinovskaya (2012) found that in the human fetus already at the 10th week during the intensification of the processes of differentiation of immunocytes, IFN-γ begins to be determined, the concentration of which significantly increases by 22 weeks of gestation and is determined in the blood and tissues of the fetus until the spleen cells do not begin to synthesize immunoglobulins, which confirms the protective role of IFN in early embryogenesis. Moreover, IFN was not detected in the blood of healthy mothers during this period, which was the evidence of its embryonic origin and autonomous functioning of the fetal interferon system.
There are reports (Kushnareva et al., 2016) about the synthesis of IFN-α in fetal tissues from the 10th week of gestation, when the first cells of the lymphoid series are found in the thymic epithelial rudiment, the processes of cell and tissue differentiation are intensified, and with their maximum proliferative activity, IFN-α is detected in all organs (lungs, heart, intestines, skin, muscles, brain).
Considering the variety of interferon functions, the lack of knowledge about them in agricultural animals, the studies of the cytokine profile in piglets in the early postnatal period, are very relevant.
The aim of this work was to study the level of cytokines in piglets in the early postnatal period, taking into account their content in colostrum (milk) of sows.
MATERIALS AND METHODS
The studies were carried out in February and March 2019 on piglets obtained from cross-breed sows (Large White + Landrace + Duroc) of the third farrow on the industrial pig breeding farm of Co Ltd “Vishnevskoe” in Verkhne-Khavskiy rayon of Voronezh region. The animals were kept under optimal microclimate parameters, taking into account their physiological state. During the experiment, sows were fed with SK-2 mixed fodder, according to the manufacturer, balanced in energy, protein, amino acids, vitamins, macro and microelements.
Piglets were fed with prestarter feed from 5-7 days of life.
Clinical observations of sows and piglets obtained from them were carried out until weaning of the piglets at the age of 28 days.
Piglets were sampled from the orbital venous sinus after ketamine anesthesia. All manipulations with animals were carried out in compliance with the international rules for conducting experiments on animals- Protocol No. 4 of 2019 “Commission for the Control of the Maintenance and Use of Experimental Animals” (Commission on Bioethics) of the Federal State Budgetary Scientific Institution “All-Russian Research Veterinary Institute of Pathology, pharmacology and therapy”.
Determination of cytokine content
Blood serum content of IL-1β, IL-2, IL-4, IL-10, TNF-α, IFN-γ in piglets (n = 6) before colostrum and at the age of 1, 7, 14, 22, 26 days and in colostrum (milk) of sows (n = 6) after farrow on days 1, 7, 14, 22, 26 of lactation were determined by the immune-enzyme analysis (EIAs), followed by taking into account the results obtained on the Uniplan-TM spectrophotometer in accordance with the approved guidelines for diagnostic kits (Vector-Best, Novosibirsk, Russia).
Expression of cytokine genes
To study the level of expression of IL-1α, IL-10 genes, actin proteins (reference gene), blood samples of piglets that did not suck colostrum, and colostrum were used. These samples were frozen immediately after sampling. RNA from blood and colostrum was isolated using the RNA-EXTRAN kit (Synthol, Moscow) according to the attached instructions.
Reverse transcription was performed using the MMLVRT kit (Evrogen, Russia)
Real-time quantitative polymerase chain reaction (qPCR) was performed on CFX96 Real-Time PPCR Detection System (BioRad, USA) using qPCRmix-HS SYBR + LowROX mixture (Evrogen, Russia) according to the following protocol: primary denaturation for 3 min at 95°C; 38 cycles, including denaturation for 30 sec at 95 °C, annealing of primers for 30 sec at 58 °C, elongation of the chain for 30 sec at 72 °C, signal registration.
The threshold cycle values were determined using Bio-Rad CFX Manager 3.1 software (Bio-Rad, USA). The relative level of expression of the studied genes was carried out using 2 -∆∆Ct method (Livak and Schmittgen, 2001).
To study the levels of cytokine gene expression, primers were developed: direct CAAGGCACGTGTGGTGATGG, reverse CATCATTCAGGATGCACTGG for amplification of IL1α; direct CTGAACAGCTGCATCCAC, reverse CTCTCTGCCTTCGGCATTAC for amplification of IL0. The actin gene was used as the reference gene, for which the primers were previously developed (Galindo et al., 2012).
Statistical analysis of the obtained data was carried out using the program statistica v6.1, reliability assessment by Student Criterion.
RESULTS AND DISCUSSION
During the period of immunological studies in sows and piglets, indicators of clinical status were within normal limits.
Cytokine levels in colostrum (milk) of sows
When studying the level of cytokines in colostrum (milk) of sows, it was found (Table 1) that they had a high content of IL-1β, IL-2, IL-4, IL-10, TNF-α and IFN-γ after farrow. They can be produced and secreted in the mammary gland, have immunomodulating activity and affect the immunity of the newborns (Hagiwara et al., 2000). A decrease in the level of studied mediators in the colostrum of sows a day after farrow by 7.4%, 11.6, 6.3, 5.6, 5.3 and 11.4%, respectively, was recorded and it was associated with the transfer of them to the newborn piglets.
In the subsequent periods of studies, the daily parameters of IL-1β and IL-10 in sows’ milk were maintained with slight fluctuations throughout the entire lactation period, as well as the levels of IL-2 and TNF-γ up to 22 days, which underwent changes on day 26, the amount of IL- 2 decreased by 17.1%, and TNF-α increased by 27.8%.
The content of cytokines IL-4 and interferon-γ significantly changed in sows’ milk during lactation.
The amount of IL-4 secreted by T-lymphocytes (type II helpers) and regulating the formation of humoral immunity increased on the 7th and 14th days compared with the daily parameters by 7.5 and 54.7%, respectively, and on the 22th and 26th days it was lower by 7.4 and 36.0% than two-week value.
The dynamics of changes in the content of IFN-γ, which is a product of type I T-lymphocytes - helpers and possessing the most pronounced immunomodulatory properties, in colostrum (milk) of sows was similar to that in IL-4.
On the 7th and 14th days of sows’ lactation, its level in milk was by 11.1 and 45.1% higher than the daily parameter, and on the 22nd and 26th days it decreased by 18.8 and 18.6% compared with the previous value.
When studying the expression levels of the IL1a, IL10, and act genes in sow colostrum, no logarithmic amplification curves were observed, which indicates the absence of cDNA (mRNA) in the studied colostrum samples, which is apparently associated with the presence of a large amount of fat in it or the absence of living cells, of which enough mRNA can be isolated.
The content of cytokines in the serum of piglets
Before colostrum intake, piglets had a relatively high level of cytokines, which at one day of age tended to increase IL-2 by 25.0%, IL-4 by 54.5; IL-10 - by 7.5; TNF-α by 5.7 and IFN-γ by 7.9% (Table 2).
In the subsequent periods of research, the piglets showed insignificant changes in the content of pro-inflammatory cytokines IL-1β and TNF-α, mediators of the primary immune response, which play a major role at the early stage of the inflammatory process (Table 2).
The amount of IL-1β slightly (from 1.0 to 7.2%) increased in animals on the 7th-26th days compared with that before colostrum, and TNF-α only on the 7th day of life (by 8.6 %).
An insignificant increase in these proinflammatory cytokines in piglets is apparently associated with the effect of the technological stress factors of a non-infectious origin on the body.
Table 1: Cytokine content in colostrum / sows’ milk.
|
Parameters |
Period of the studies (twenty-four hours after farrow) |
|||||
| 0 | 1 | 7 | 14 | 22 | 26 | |
|
IL-1β |
15.9±0.06 |
14.8±0.11*** |
15.4±0.68 | 16.1±0.53 | 15.5±0.16 | 15.2±0.16 |
| IL-2 | 19.3±0.28 |
17.3±0.41*** |
14.5±0.62ххх |
17.3±0.92х |
17.0±0.97 |
14.1±0.22хх |
| IL-4 | 20.1±0.27 | 18.9±1.96 | 21.6±1.12 | 31.1±2.87 | 28.8±2.49 |
19.9±1.29хх |
| IL-10 | 24.6±0.18 |
23.3±0.49* |
21.9±0.63х |
22.6±0.25 | 22.8±0.36 | 23.0±0.55 |
|
TNF-α |
4.0±0.38 | 3.8±0.13 | 3.5±0.04 | 3.6±0.09 | 3.6±0.08 |
4.6±0.35хх |
|
IFN-γ |
91.6±0.98 |
82.2±0.39*** |
91.3±2.13ххх |
119.3±7.07ххх |
96.9±4.89хх |
78.9±4.29хх |
Note: *Р<0.05; **Р<0.001; ***Р<0.0001 relative to the period before farrow; х Р<0.05; хх Р<0.001; ххх Р<0.0001 relative to the previous period.
Table 2: Cytokine profile in piglets in the early postnatal period.
|
Parameters, pg/ml |
Age (days) |
|||||
| 0 | 1 | 7 | 14 | 22 | 26 | |
|
IL-1β |
10.9±0.02 | 10.9±0.20 | 11.7±0.61 | 10.95±0.13 | 11.0±0.11 | 11.1±0.41 |
| IL-2 | 9.2±0.77 |
11.5±0.49* |
11.4±0.46* |
7.0±0.14ххх |
7.9±0.64ххх |
17.1±0.14ххх |
| IL-4 | 3.3±0.29 |
5.1±0.33*** |
2.95±0.03ххх |
2.8±0.05ххх |
2.7±0.025ххх |
3.2±0.19х |
| IL-10 | 19.9±0.19 |
21.5±0.75* |
20.5±0.25 | 19.9±0.06 | 20.1±0.126 | 20.2±0.13 |
|
TNF-α |
3.5±0.08 | 3.7±0.09 |
3.8±0.12* |
3.7±0.041* |
3.5±0.063 | 3.5±0.17 |
|
IFN-γ |
78.3±2.21 | 84.5±5.91 |
102.7±3.97*** |
133.6±4.71*** |
138.1±5.18*** |
117.6±3.51*** |
Note: *Р<0.05; **Р<0.001; ***Р<0.0001 relative to the period before drinking colostrum;
хР<0.05; ххР<0.001; хххР<0.000 relative to the previous period.
The amount of anti-inflammatory IL-10, synthesized by T-lymphocytes (type II helpers) and cytotoxic lymphocytes, tended to increase slightly (7.5 and 2.5%) in piglets at the age of 1 and 7 days compared with those before taking colostrum.
In contrast to slight fluctuations in the level of IL-2 in sows’ milk, significant changes occurred in piglets in the content of this cytokine produced by type I T-helper cells and cytotoxic T cells and determining the development of cellular immunity.
Its level in suckling pigs at the age of twenty-four hours increased compared with that before obtaining colostrum by 25.0%, on the 7th day - by 23.9%, which was associated with the intake of interleukin-2 with colostrum (milk). Subsequently, there was a decrease in its amount on the 14th day by 38.6%, the 22th by 30.7%, and on the 26th day, despite a decrease in the content of sows’ milk, it increased by 2.2 times, because it was synthesized by the cells of the own immune system that responded to microorganisms circulating in the animal habitat.
The blood serum amount of IL-4 in piglets after an increase by 54.5% at the age of twenty-four hours decreased on the 7th, 14th, 22th days by 41.7, 45.1 and 47.1%, respectively, and on the 26th day its increase by 18.5%, compared with the previous parameters, was recorded. It was associated with the synthesis of the cytokine by the cells of the own immune system.
The level of interferon-γ was high in piglets before colostrum intake, which indicated its synthesis by the fetal cells, apparently associated with the effects of parvovirus, circovirus type II, etc., circulating on all pig breeding farms. Their transplacental transfer promotes their spread in the endometrium, placenta, and various organs of the fetus (Nauvinn, 2013). The high content of IFN-γ, along with the participation in the development and differentiation of fetal and newborn cells (Malinovskaya, 2012), is apparently also aimed at ensuring the adaptation of the latter to the conditions of extrauterine development.
During the suckling period, the content of interferon-γ compared with that in piglets before colostrum intake increased at the age of twenty-four hours by 7.9%, on the 7th day by 31.2%; on the 14th - by 70.6%; on the 22nd – by 76.4%, and on the 26th day - by 15.2%, which was associated with receiving it with colostrum (milk) of sows and the synthesis by the cells of the own immune system, especially when the amount of IFN-γ in milk decreased at the end of sows’ lactation.
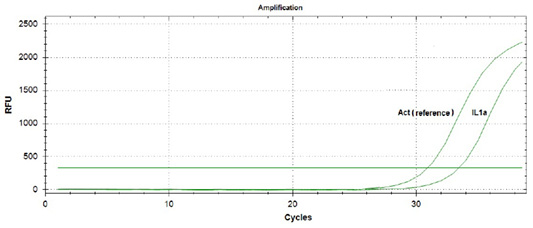
During amplification of the protein genes of the studied cytokines, expression of the interleukin 10 gene in the blood of piglets that did not suck colostrum was not detected. At the same time, the expression of interleukin 1α gene was observed (Figure 1).
Thus, the registration of the IL-1α gene and the presence of the cytokines IL-1β, IL-2, IL-4, IL-10, TNF-α and IFN-γ in the blood serum of piglets before colostrum intake indicate their synthesis by fetal cells, the level of which in newborns, it increases due to the intake from colostrum (milk) of sows and the synthesis of cells of their own immune system.
The advantage of the conducted studies is the study of the cytokine profile in piglets in dynamics, since there are no data in the available literature.
CONCLUSIONS and Recommendations
In colostrum of sows after farrowing, a high content of cytokines IL-1β, IL-2, IL-4, IL-10, TNF-α and IFN-γ was found, which decreases after a day and with small fluctuations the level of IL-1β and IL-10 remains in milk during the entire lactation period, and IL-2 and TNF-α up to 22 days, followed by a tendency to decrease the amount of IL-2 and increase the content of TNF-α on the 26th day, respectively.
The level of IL-4 and IFN-γ in comparison with the daily parameters increases on days 7 and 14 with a decrease in their content at the end of lactation in sows.
The expression of IL-1α cytokine gene and the presence of IL-1β, IL-2, IL-4, IL-10, TNF-α and IFN-γ were recorded in the newborns prior to colostrum, indicating their synthesis by fetal cells, the number of which in piglets at the age of twenty-four hours increased due to their intake with colostrum. The daily parameters of IL-1β, IL-10 and TNF-α with small fluctuations are maintained in piglets throughout the entire suckling period.
The content of IL-2 in piglets during the first week of life and IL-4 at the age of twenty-four hours significantly increases with a subsequent decrease to days 14 and 22, respectively, and by the end of the suckling period, despite a decrease in the number of cytokines in milk, it increases because they are synthesized by the cells of the own immune system.
The level of IFN-γ in piglets increases during the entire period of suckling, despite a significant decrease in its amount in milk, at the end of lactation of sows, which indicates the synthesis of cytokine by cells of their own immune system.
The data obtained are recommended for use in assessing the immune status of healthy piglets and piglets with pathology, completeness of recovery of animals, prophylactic and therapeutic efficacy of immunomodulating drugs. In future studies, it is planned to study the cytokine profile in older piglets, the influence of technological stress factors on it (early weaning, transfer to nursery and fattening).
Acknowledgements
This work was supported by the Presidential Grant for State Support of Leading Scientific Schools (research agreement NSh-2535.2020.11).
Novelty Statement
The expression of the IL-1α cytokine gene in the blood and the presence of IL-1β, IL-2, IL-4, IL-10, TNF-α and IFN – γ cytokines in the serum of milk-free piglets was shown, which indicates their synthesis by fetal cells. Dynamic changes of these mediators in piglets during the entire period of their keeping under sows are shown.
Author’s Contribution
AS: Experiment idea and design. EM & MZ: methodology. MA, KK & MS: conducted the experiment. AS, LS, MS, EM & MZ: writing and editing the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES