The Journal of Advances in Parasitology
Research Article
Ancylostomiasis, Giardiasis and Isosporiasis in a Domestic Short Hair Cat in Kota Bharu, Malaysia
Hasna Nadia Hasan Sazalli, Intan Noor Aina Kamaruzaman, Mohamed Reza Mohamed Tarmizi, Ibrahim Abdul-Azeez Okene, Rumaizi Shaari, Pwaveno Huladeino Bamaiyi*
Department of Clinical Studies, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Beg Berkunci No 36, Pengkalan Chepa - 16100, Kota Bharu, Kelantan, Malaysia.
Abstract | A 6 months old domestic short hair cat named “Mok Tam” was presented to the Veterinary Clinic of the Universiti Malaysia Kelantan (KVUMK) with the complain of inappetance and vomiting. The history showed that the faeces was greenish-mucoid for 3 days before it was presented to KVUMK. On 3rd October 2014, “Mok Tam” was inappetant, dull and depressed. The owner noticed that Mok Tam was having watery diarrhea with brown yellowish faeces. After 4 days later, Mok Tam was still inappetant and there was presence of pasty greenish diarrhoea and vomiting. The cat was presented to KVUMK on 10th October, 2014. Upon physical examination, Mok Tam was dull and mentally depressed. The body weight was 1.3kg and temperature was 37oC (hypothermia), the heart rate was 108bpm which is lower than normal range indicative of bradycardia, and respiratory rate was normal. The Capillary refill time (CRT) and dehydration status was normal but the mucous membrane was pale. Further diagnostic workup was conducted and this was a case of Ancylostomiasis, Giardiasis and Isosporiasis. The cat recovered a week after therapy was instituted.
Keywords | Ancylostomiasis, Giardiasis, Isosporiasis, Domestic Short Hair Cat, Malaysia
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | December 30, 2015; Revised | March 23, 2016; Accepted | March 28, 2016; Published | April 22, 2016
*Correspondence | Pwaveno Huladeino Bamaiyi, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa, 16100, Kota Bharu, Kelantan, Malaysia; Email: pwaveno.hb@umk.edu.my
Citation | Sazalli HNH, Kamaruzaman INA, Tarmizi MRM, Okene IAA, Shaari R, Bamaiyi PH (2016). Ancylostomiasis, Giardiasis and Isosporiasis in a domestic short hair cat in Kota Bharu, Malaysia. J. Adv. Parasitol. 3(3): 75-80.
DOI | http://dx.doi.org/10.14737/journal.jap/2016/3.3.75.80
ISSN | 2311-4096
Copyright © 2016 Sazalli et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Ancylostoma spp. Belong to the phylum nemathelminthes, order-nematoda and ancylostomatoidea family. Ancylostomiasis is a zoonotic disease of dogs and cats caused by parasites belonging to the genus Ancylostoma which includes species such as: Ancylostoma braziliense (mostly in cats), A. ceylanicum (in cats and dogs), A. tubaeforme (cats), A. duodenale (mainly human pathogen) and A. caninum (in dogs) (Brooker et al., 2008; Traub, 2013). Hookworms which also include members of the genera, Necator and Uncinaria, affect over 400 million people around the world causing stunted growth, contact dermatitis, anaemia and impoverishing the communities (Brooker et al., 2008; Jonker et al., 2012; Alipour and Goldust, 2015; Schwarz et al., 2015). In dogs and kittens it leads mainly to anaemia and enteritis which is more severe in puppies and kittens (Snyder and Wiseman, 2012; Riggio et al., 2013; Dracz et al., 2014). Hookworm infection can cause cutaneous larvae migrans or eosinophilic enteritis in human with signs of serpentine and pruritic lesion (Supplee et al., 2013; Rajesh et al., 2014).
Giardia spp. belong to the protozoa phylum under the order-sarcomastigophora and mastigophora family. Giardiasis is a chronic, intestinal flagellated zoonotic protozoan infection that is seen worldwide in most domestic mammals causing bowel diarrhoea especially affecting the duodenum in dogs and cats (Ballweber et al., 2010; Gruffydd-Jones et al., 2013).
Isospora spp. is a protozoan under the order-apicomplexa and coccidia family. They are commonly found coccidian parasites in dogs and cats (Riggio et al., 2013; Yang and Liang, 2015) which can cause zoonotic isosporiasis (Nisar et al., 2009). Isosporiasis is usually an acute invasion and destruction of intestinal mucosa of the colon by protozoa of the genera Isospora leading to watery diarrhoea which may sometimes turn bloody, vomiting, mental depression and ultimately death in severely infected dogs especially if immunocompromised (Mitchell et al., 2007; Barutzki and Schaper, 2013).
Ancylostoma spp. have hook-like structures with 3 pairs of teeth and the eggs are elliptical and thin-shelled. Transmission may result from ingestion of infective larvae from the environment or can also result from larval invasion through the skin. Infective stage larvae ingested by the cats moults to L4 and migrate via lungs or directly to the intestine and mature to adult. Adult worms may lay eggs in small intestine which are excreted together with the faeces. In a favourable environment, eggs then hatch into L1 and undergo two moults into L2 and L3 (Bowman et al., 2010).
Giardiasis infection is caused by G.lamblia and G.duodenalis. Giardia spp. occurs in two forms, the trophozoite and the cyst. The trophozoite have broad anterior and it is flat with 4 pair of flagella while cysts is ovoid in shape and has thick wall with 4 nuclei inside the cyst. Giardia spp. can be transmitted via ingestion and can be waterborne. After ingestion of the the Giardia spp. cysts, gastric acid and pancreatic enzyme exposure in the duodenum induces release of the two trophozoites, which mature quickly and attach to the brush border of villous epithelium. In cats and dogs that have infection of Giardiasis signs of diarrhea with greenish tinge and mucus and foul smelling may be noticed (Gruffydd-Jones et al., 2013; Tysnes et al., 2015).
Isosporiasis is caused by I. felis and I. rivolta Isospora spp. has three stages which are schizonts with small rounded cells, gamonts with single ovoid nucleus and oocyts which are ovoid to elliptical in shape (Zajac et al., 2012). Infection by Isospora spp. in dogs or cats is initiated by ingestion of sporulated oocysts in the environment. Sporulated oocyst has 2 sporocysts and each containing 4 sporozoites. The oocysts invade the intestinal mucosa or epithelial cells in other locations and develop intracellularly into multinucleated schizonts. Each nucleus develops into an infective body called merozoite which then enter new cells and repeat the process. The merozoites develop into either macrogametocytes (females) or microgametocytes (males). These produce a single macrogamete or a number of microgametes in a host cell. After being fertilized by a microgamete, the macrogamete develops into oocysts. The oocysts have resistant wall and are discharged unsporulated in the faeces. The clinical signs can be seen in cats are watery to mucoid, sometimes blood tinged diarrhea. Depending on the age of the animal and the parasite burden, severe dehydration and death can occur (Spurgeon, 2012).
There is paucity of literature on concurrent infections with ancylostomiasis, giardiasis and isosporiasis in Malaysia. This study reports a concurrent infection of ancylostomiasis, giardiasis and isosporiasis in a domestic short hair cat in Kota Bharu, Kelantan state of Malaysia and elucidates the implication for animal and public health. Clinicians should watch out for such concurrent infections and treat them accordingly to safeguard animal and public health.
HISTORY
On 3rd October 2014, a 6 months old female domestic short hair cat named “Mok Tam” was found to be inappetant, dull and depressed by the owner, who also noticed that it was having watery diarrhoea with brown yellowish faeces. By 7th October the cat was still inappetant and there was presence of pasty greenish diarrhoea and vomiting. After 3 days later on 10th October 2014, it was presented to the Veterinary Clinic of Universiti Malaysia Kelantan (KVUMK). The cat weighed 1.3 kg and vaccination and deworming status was unknown. Owner complain was inappetance, vomiting and greenish mucoid faeces for 3 days before it was presented to KVUMK.
PHYSICAL AND CLINICAL EXAMINATION
Physical examination was done on “Mok Tam” and we found that the temperature was lower than normal range indicating hypothermia and heart rate was also lower than normal range indicative for bradycardia. Mucus membrane of gum was pale, indicating anaemia. Capillary Refill Time (CRT) was 2 seconds and dehydration status was 5%. The cat also showed sign of dullness and depression in addition to inappetance. Based on history, physical examination and clinical findings, the following differential diagnoses were ruled in:
- Food allergy and foreign body based on vomiting
- Feline panleukopenia
- Ancylostomiasis, toxocarosis, toxoplasmosis, Giardiasis, Isosporiasis and salmonellosis based on diarrhoeal signs
- Haemoparasitism due to pale mucous membrane
- Mycoplasmosis, immune-mediated haemolytic anaemia (IMHA), autoimmune haemolytic anaemia (AIHA)
Based on history and physical examination, blood parasites can be ruled out due to absence of ticks on the cat. The tick commonly found in cats is Ixodidae. For the diagnostic work-up, feline parvovirus test was done by using feline parvovirus test kit. The faecal sample was taken and the cat was tested for parvovirus infection. Then, complete blood count and serum biochemistry was done. Blood from cephalic vein was collected into EDTA tube and plain tube. The blood sample was sent to the laboratory for the complete blood count and serum biochemistry test.
Faecal smear and faecal floatation test was done using faecal sample taken from the rectum. The slide was examined under a light microscope (10x and 40x magnifications) to determine the presence of parasitic worm eggs. The identity of the eggs found was confirmed in the Parasitology laboratory of Faculty of Veterinary Medicine Universiti Malaysia Kelantan.
RESULTS
The feline parvovirus test was negative indicating that “Mok Tam” was not having parvovirus infection. For the complete blood count result there was reduction of the level of RBC and haemoglobin but high level of mean corpuscular volume (MCV) with low mean corpuscular haemoglobin concentration (MCHC) indicating microcytic hypochromic anaemia. There was also leucocytosis, lymphocytosis, granulocytosis and thrombocytopenia. Increased level of granulocytes indicated that there was active infection within the body.
Serum biochemistry showed that there was slight reduction in the level of urea indicating low appetite and low protein production. It also showed hypoglobulinemia, hypoalbuminemia and hypoproteinemia suggestive of ongoing haemorrhage or inflammation of the tissue.
Fecal smear was done by using a drop of glycerol, the result was positive for Ancylostoma spp. (Figure 1).
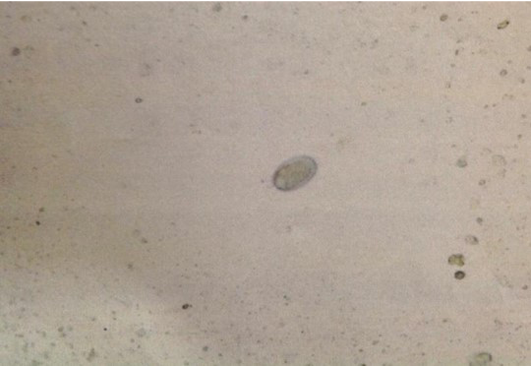
Figure 1: Ancylostoma spp. egg
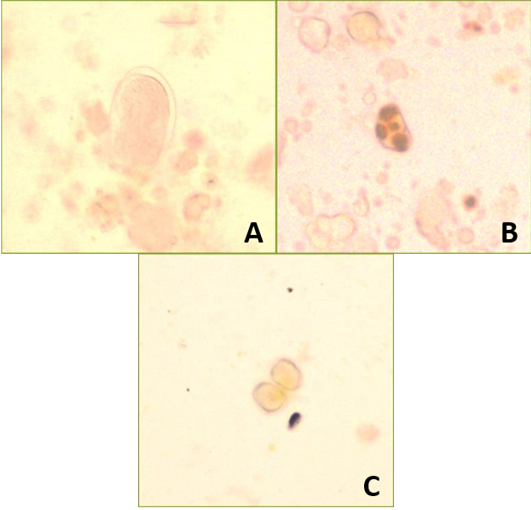
Based on fecal floatation, three difference eggs or oocysts was found which are Ancylostoma spp., Giardia spp. and Isospora spp. (Figure 2A, B and C respectively).
A) Ancylostoma spp.; B) Giardia spp.; C) Isospora spp.
Ancylostoma eggs was identified by thin shell and ovoid shape with presence of 8-16 morula while Giardia cysts is identified with thick shell and presence of 4 nuclei inside the cysts and Isospora oocysts was identified by presence of two sporozoites (Zajac et al., 2012).
For the differential diagnoses, toxocariasis can be ruled out due to the absence of Toxocara eggs during fecal floatation test and fecal smear test. Blood parasites also can be ruled out due to the absence of any tick during careful physical examination. Then, immune mediated hemolytic anemia (IMHA) and autoimmune haemolytic anemia (AIHA) can be ruled out because usually in these cases there will be increased MCHC level due to extravascular anaemia, due to haemolysis of the red blood cells but in this case MCHC is lower. This can be supported further by the serum biochemistry where the level of bilirubin should be higher in cases of AIHA and IMHA but in this case, the bilirubin was normal (Vaden et al., 2011).
Based on history, clinical findings, and diagnostic finding, it was confirmed that this cat was suffering from Ancylostomiasis, Giardiasis and Isosporiasis. The cat was warded at the clinic facility for 1 week to facilitate proper treatment and monitoring of its progress.
TREATMENT AND DISCUSSION
“Mok Tam” was treated with Metronidazole 2.6 ml twice a day for 5 days via slow IV injection. Metronidazole acts as antibacterial and antiprotozoal and can be used to treat Giardia and other protozoa infection. Next, it was treated with antibiotic, Amoxycillin Clavumac Acid 1ml twice per day for 7 days per os. Then, since it was having diarrhea, Kaolin Pectin was given as gastrointestinal tract protectant per os 2 ml twice per day for 5 days. Pectin is used to aid absorption in intestinal lumen and form galacturonic acid that will decrease the pH level in intestine and pectin also can acts as absorbent which it can absorb the entire toxin in intestine (Hsu, 2013) (Table 1).
Table 1: Medication regime given
|
Drugs |
Dosage |
Dose given |
Route & Regime |
|
Metronidazole |
8-10 mg/kg |
2.6 ml |
IV BID for 5 days |
|
Amoxycillin (Clavumac Acid) |
12.5-25 mg/kg |
1 ml |
PO BID for 7 days |
|
Kaolin Pectin |
3-6 mg/kg |
2 ml |
PO BID for 5 days |
|
Loperamide |
0.2 mg/ml |
0.13 ml |
PO BID for 3 days |
|
Praziquantel + Pyrantal pomoate (Drontal®) |
1 tab/4kg |
½ tab |
PO once |
|
Vitamin B complex |
- |
1 tab |
PO BID for 5 days |
|
i/d diet |
- |
- |
PO BID for 5 days |
|
Sodium hypochloride (Normal Saline) |
- |
- |
IV for 2 days |
Loperamide was given 0.13 ml twice a day orally for 3 days. Loperamide acts as anti-diarrheal agent and used as gastrointestinal motility modifier which Loperamide can decrease the intestinal secretion (Plumb, 2008). Anthelmintic was also given since there was no history of deworming. Drontal that consists of the active ingredient of praziquantel and pyrantel pomoate was given half tab, once orally. Praziquantel was effective to kill the parasites especially nematodes by increase calcium ion flux into the worm while pyrantel acts as depolarizing and neuromuscular blocking agent which can paralyse the worm (Hsu, 2013).
Vitamin B complex was given as nutritional supplement and i/d diet was given as digestive health support. These were given twice a day for 5 days orally. The sodium hypochloride (normal saline) was given IV to replace all the fluid and mineral deficits due to vomiting and diarrhoea.
DISCUSSION
Infections with these 3 parasites are common in different parts of the world in cats and zoonotic transmission to humans (Murugan et al., 2015). Cats with Ancylostoma infection usually will have microcytic hypochromic anaemia due to blood loss because the Ancylostoma spp. takes in the blood of the cat in the intestines, and the worm will migrate into the wall of the intestine while some of the worms would travel to lungs and trachea which would lead to vomiting and respiratory signs such as coughing. At the wall of the intestine the parasite will suck the blood and release anticoagulant which leads to diarrhea with blood tarry faeces due to the blood clot that the worm sucked from the intestine of the cat (Schaer, 2009).
In addition, the Ancylostoma spp. would cause inflammation and haemorrhage in the intestine and cause hypoproteinemia and anaemia which were seen in this case. The anaemia is responsible for the pale mucus membrane which was seen during physical examination.
The migration of the worms to the trachea will lead to focal eosinophilic granulomatous reaction and cause tracheitis leading to tissue damage and hyperkalemia occurring with hyponatremia and finally causing bradycardia (Bowman et al., 2010; Montgomery 2013; Ueda et al., 2015) which was seen in this case.
Isospora spp. will directly go to the colon and invade the intestinal mucosa which causes destruction of the intestine and cause malabsorption leading to watery diarrhea. For giardiasis, usually the Giardia spp. will cause destruction in duodenum and the trophozoite will attach at the brush border of the villi epithelium and cause irritation and mucosal layer injury leading to villi atrophy and sloughing off and finally leading to increase in proliferation of crypt cell (Capári et al., 2013; Riggio et al., 2013). This process will cause hypersecretion by the goblet cell and lead to increase mucus secretion formation, as in this case.
Treatment approach in this case involved therapy and supportive care. Supportive care may include fluid therapy, iron supplements and blood transfusion. For fluid therapy, it is important to replace the fluid that was lost due to vomiting and diarrhea and also to stabilize the cat. Iron supplement and blood transfusion should be considered in chronically ill cats where the anaemia is very severe and they are recumbent.
There are many choices of drugs that can be used in order to treat Hookworm infection, Giardia and Isospora. For Ancylostoma, Macrolidex, Benzimidazoles and Tetrahydropyrimidines group of drugs can be used. Macrolidex group of drugs consist of Ivermectin, Selamectin and Milbemycin which are effective against nematode and arthropod and have broad spectrum activity and effective at low dose. Benzimidazoles consist of Febantel and Febendazole which are effectively against Ascarids, Hookworms and Whipworms in both adult and larvae forms and they can inhibit nematode parasites within one hour of administration. The treatment of choice for hookworm is Tetrahydropyrimidines such as Pyrantel (Plumb, 2008).
The treatment of giardiasis can be done with Furazolidone and Fenbendazole while for isosporiasis, Sulfadimethoxine and Amprolium are potent and efficacious (Plumb, 2008).
A summary of the treatments of choice to overcome these infections are shown in Table 2. The role of good management and hygiene in preventing parasitic infections should be emphasized to pet owners. Pet owners should avoid giving contaminated water and feed to their pets. Sick cats should be isolated from other cats and humans by keeping them in cages individually especially when they show any signs of diarrhea or vomiting to prevent the disease from spreading to other cats and humans. In addition, disinfection and cleaning of the cages or transport container with suitable agents such as diluted sodium hypochlorite will ensure destruction of parasites or their eggs (Parnes, 1997).
Table 2: Summary of some treatment of choice for hookworms, Giardia and Isospora
|
Drugs |
Hookworms |
Giardia spp. |
Isospora spp. |
|
Pyrantel pamoate |
X |
||
|
Fenbendazole |
X |
X |
|
|
Furazolidone |
X |
X |
|
|
Milbemycin |
X |
||
|
Metranidazole |
X |
||
|
Sulfadimethoxine |
X |
||
|
Amprolium |
X |
Good client education is important because they may not be aware of the risks posed by zoonotic diseases. They also need to be educated to bring their pets for deworming, vaccination and other veterinary care periodically. They should be made aware of the potentials of transmission of these infections to other animals especially with outdoor cats. They also need to ensure that their pets are cleaned and groomed regularly.
CONCLUSION
Ancylostomiasis, giardiasis and isosporiasis are common intestinal tract diseases in cats. “Mok Tam” was diagnosed with these three and managed with anthelminthic, anti-protozoal, multi-vitamin and anti-diarrhoeal regime. The prognosis was good and the cat recovered after a week of intense therapy. The zoonotic implications of these parasites call for caution on the side of the veterinary clinician and the client to minimize the risk of exposure and infection.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
The authors would like to thank the staff of the Faculty of Veterinary Medicine Universiti Malaysia Kelantan and the cat owner for their cooperation.
AUTHOR’s CONTRIBUTION
All the authors contributed significantly to this paper and are in agreement with the contents. Authors also agree with the conditions of the copyright of the journal.
REFERENCES