Journal of Animal Health and Production
Research Article
Protective Effect of Cholestyramine and Oxihumate on Experimentally Induced -Ochratoxicosis in Broiler Chickens
Abdel Moneim A. Ali1, Mahmoud F. Fahmy1, Mohamed M. Metwally1, Hend A. Azazy2, Rehab E. Mowafy2*
1Pathology Dep. Faculty of Veterinary Medicine - Zagazig University; 2Animal Health Research Institute (ACR), Dokki, Gizza, Egypt.
Abstract | Despite its less prevalence compared to other mycotoxins, ochratoxin-A (OTA) is considered the most powerful toxin causes avian ochratoxicosis. Therefore, the aim of this study was to evaluate the protective role of antimycotoxins cholestyramine and oxihumate against the toxicity of OTA. To fulfil that, one-day-old chicks (n=64) were randomly allocated into four groups (16 chicks per group); group-1(basal diet + ochratoxin-A), group-2 (basal diet + ochratoxin-A + cholestyramine), group-3 (basal diet + ochratoxin-A + oxihumate), and group-4 (basal diet). After 36 days, livers, kidneys and muscles were harvested for histopathological studies as well as residual analysis of OTA. Nephrotoxicity was the most detected lesion among chickens of group-1. The intensity of the lesions-related toxicity was significantly (P˂0.05) reduced in the treated groups (group-2 and group-3). OTA residues were significantly reduced in the kidneys (P˂0.05), livers (P˂0.05), and muscles (P˂0.05) in both treated groups in comparison with that of group-1. In conclusion, both cholestyramine and oxihumate ameliorate the tissue deteriorations of OTA in chickens, whereas cholestyramine was more effective than oxihumate.
Keywords | Ochratoxin, Cholestyramine, Oxihumate, Mycotoxicosis, Chicken
Received | July 21, 2021; Accepted | August 08, 2021; Published | August 25, 2021
*Correspondence | Rehab E Mowafy, Animal Health Research Institute (ACR), Dokki, Gizza, Egypt; Email: [email protected]
Citation | Ali AMA, Fahmy MF, Metwally MM, Azazy HA, Mowafy RE (2021). Protective effect of cholestyramine and oxihumate on experimentally induced -ochratoxicosis in broiler chickens. J. Anim. Health Prod. 9(s1): 62-68.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.s1.62.68
ISSN | 2308-2801
Copyright © 2021 Mowafy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Mycotoxins are metabolites of filamentous fungi and have strong economically negative impact on livestock, poultry and humans (Ropejko and Twarużek, 2019). The disease evolved from mycotoxin-contaminated animal feed, as a global issue, ranged from acute disease outbreaks to severe metabolic disorders (Bryden, 2012; Murugesan et al., 2015). The mycotoxins in the worldwide also have health risks that vary from allergic reactions to deaths on animals and humans (Haque et al., 2020).
Ochratoxin-A (OTA) is one of the mycotoxins most commonly isolated from the environment. It is regularly detected in cereals, coffee, cocoa, wine, beer, spices and milk, but also occurs in the other foodstuffs. Accordingly, OTA is also found in human body fluids like urine, breast milk and blood. This occurrence was associated with increased level of OTA exposure (Ropejko and Twarużek, 2019). OTA is considered as a powerful avian nephrotoxin, although the mode of action of ochratoxin is not yet understood and appears to be very complicated (Pfohl-Leszkowicz et al., 2007). Protein inhibition, NDA adduct constituent, induction of oxidative stress in addition to necrosis /apoptosis and cell cycle arrest play role in its toxicity (Tamas and Miklós, 2016). OTA induces enlarged, pale kidneys, degenerative, necrotic, and inflammatory changes subsequently impairing its function in addition to it causes hepatic lesion, lymphoid atrophy together with depletion of lymphocytes (Stoev et al., 2004; Koynarski et al., 2007; Patial et al., 2013) .
Cholestyramine (CH) is an effective binder for OTA in vitro although it has been used primarly as bile acids adsorbent in the gut to reduce cholesterol (Avantaggiato et al., 2005). The treatment of the intoxicated chicks with cholestyramine in the drinking water showed partially and temporarily improvement in the relative weights and the histopathological lesion scores of the tested immune organs (spleen and bursa of Fabricius) (Abdelnaser et al., 2017).
Oxihumates are the salts of humic acid in which the exchange site is Ca+ , Na+ , Al+ and Fe+2 rather than hydrogen. Humic substance able to inhibit bacterial and fungal growth, thus decreasing levels of mycotoxins in feed (van Rensburg et al., 2006). Its beneficial effects include stress management, anti-inflammatory activity, antiviral properties, and prevention of intestinal diseases, mainly diarrhea in both humans and animals. As well, it improves the health status through working against pathogens, developing immunity and improved growth of broilers by increasing the digestion of protein and improved trace element utilization. It has been believed that the oxihumates have a high adsorbent capacity for several mycotoxins such as OTA (Jansen-van, 2005). The objective of this study was therefore to compare the protective effect of both cholestyramine and oxihumate for OTA toxicity in broiler chickens.
MATERIAL AND METHODS
Preparation of ochratoxin-A (OTA)
Prepared Aspergillus ochraceus strain was obtained from the Central Laboratory of Residues of Agricultural Product, Agriculture Pesticides Residues Centre, Dokki, Egypt. Aspergillus ochraceus was grown on malt peptone (MP) broth using 10% (v/v) of malt extract (Brix 10) and 0.1 % (w/v) bacto peptone (Difco) in 2 mL of medium in 15 mL tubes. The cultures were incubated at 25°C for 7-days in light/darkness. The OTA extract was analyzed by (HPLC) (Samson et al., 2004). The media was sprayed on diet to obtain OTA (100 ppb).
Experimental grouping
Sixty-four, one-day-old, Cubb breed unsexed chicks were purchased from Alkahira Company (Cairo, Egypt) and kept under standard hygienic conditions in separate floor pens (1.5m x 2m each) in open sided house. Starter diets contain 22.03-22.26% crude protein with 2970.46-2978.11 Kcal ME, while finisher diets contains 18.39%-18.59% crude protein and 3176.70 Kcal ME. Starter diets were offered from 1-18 day and finisher diets were offered from 19-36 day of age. Both feed and water were offered ad-libitum. All diets were formulated to cover the nutrient requirements (NRC, 1994). All the experimental procedures were performed at Animal Health Research Institute (Zagazig Provincial Laboratory) in accordance with the recommendations and guidelines of the Institutional Animal Care and Use Committee (ZU-IACUC) of Zagazig University.
Chicks were divided randomly into 4-groups (n=16 each) ); group-1(basal diet + ochratoxin-A), group-2 (basal diet + ochratoxin-A + cholestyramine), group-3 (basal diet + ochratoxin-A + oxihumate), and group-4 (basal diet). The chemical detoxifying agent cholestyramine (Questran ®, Bristol- Myers Squibb) was used in a dose of 170 µg/ mg mycotoxin in the ration (17 µg/ kg ration) (Solfrizzo et al., 2001). Humic acid Oxihumate (El-Nasr pharmaceutical Chemical Company, Egypt) as a physical detoxifying agent was used in a dose of 3.5 gm/kg ration (Jansen-van, 2005).
During 36 days experiment peroid, the mortalities were recorded. At the end of the experiment, the clinical signs, mortalities and post-mortem lesions of dead and sacrificed chickens were recorded with sample harvesting for further investigations.
Pathological investigation
From each group, the speciemens of kidney, liver, spleen, bursa of Fabricius and intestine were collected and fixed in 10% neutral buffered formalin. The paraffin sections of 5-µm thickness were stained with Hematoxylin and Eosin staining (Survarna et al., 2013), then microscopically examined.
Residual investigation
Residues of OTA were determined in liver, kidney and muscle tissues at the end of the experiment in the ochratoxicated groups by using High Performance Liquid Chromatography (HPLC; Prominence TM, Shimadzu® Tokyo, Japan). Twenty grams aliquot of fresh chicken tissues samples were homogenized and prepared (Matrella et al., 2006). Extracted samples were passed through the immune affinity via clean-up of 1-2 drops as per previously reported method (Beg et al., 2006).
Statistical analysis
The obtained data were analyzed by SPSS software using one-way ANOVA to determine the statistical significance of differences among groups. Duncan’s test was used for testing the inter-grouping homogeneity.
RESULTS AND DISCUSSION
Chickens of OTA intoxicated group-1 were suffered from weakness, restlessness and increased water consumption.
Table 1: Experimental groups, treatments and mortalities during the whole experiment.
|
Treatments/ Groups |
Ochratoxin A 100ppb |
Cholestyramine 170 µg / mg mycotoxin |
Oxihumate 3.5 g/kg ration |
Mortalities (%) |
| Group(1) | + | - | - | 31.25%. |
| Group(2) | + | + | - | 18.75%. |
| Group(3) | + | - | + | 18.75%. |
| Group(4) | - | - | - | - |
Diarrhoea with high urate contents, poorly feathering and paleness of both comb and wattles were also observed in some cases. Table-1 showed the mortality rate of all groups. The cumulative mortality rate of group-1 was 31.25% (5 birds/group). The aforementioned clinical findings were consistent and in agreement with those reported by (Resanovie et al., 2009; Zahoor et al., 2011; Nedeljković et al., 2015; Gaheen, 2017). In addition, it is worth to mention here, the recorded paleness of the comb and wattles could be explained via the impaired utilization of dietary carotenoids caused by OTA intoxication. In addition, OTA decreased serum iron, total iron-binding capacity and transferrin percent, therefore it induces iron deficiency anaemia which considered the most common type of hypochromic microcytic anaemia Malir et al. (2016). The other signs could be attributed to the toxic immunosuppressive effect of OTA which acting as stress factor (Al-Anati and Petzinger, 2006). Furthermore, the renal toxic effect of OTA (Elaroussi et al., 2006), led to the whitish watery diarrhea that subsequently induced relatively high mortality rate (31.25%). In earlier studies, intoxication with OTA caused moderate mortality rate i.e., 20% (Gahen, 2017) however may reach up to 35.7% (Kumar et al., 2004). In group-2 and -3, no remarkable clinical signs could be observed among the majority of chickens during the whole experimental period. However, a few experimental birds of those two groups showed mild depression and diarrhea with some mortalities of 18.75% (3 birds/group). Reduction of both clinical signs and mortalities in both treated group-2 and -3 may attributed to the ameliorative effect of the either applied antimycotoxin, cholestyramine or oxihumate, respectively. Cholestyramine reduces the plasma level of ochratoxin and enhances the fecal excretion of OTA (Kerkadi et al., 1998). Oxihumate has high adsorptive properties that hinder the gastrointestinal mycotoxin absorption (Jansen-van, 2005).
The kidneys are considered as the key target organ of OTA toxicity (Ringot et al., 2006). Necropsy revealed marked kidney injuries of chickens of group-1 such as severe kidneys paleness and enlargement with urate deposition (Vencislav et al., 2007; Bozoo et al., 2008; Hameed et al., 2013; Abo El-Fetouh et al., 2016). Furthermore, variable gross findings were noticed such as enlargement of the examined liver with yellowish discoloration. The enlargement of the liver and kidney in the instance of OTA intoxication might be due to the fact that these organs considered the major role in detoxification and elimination of OTA level. OTA is known to have direct toxic action (Stoev et al., 2000) as well as high level of its accumulation in these organs (Biro et al., 2002). Hyperaemic mucosal surface of intestine was also noticed in some cases. Some chicken showed thickening of intestinal wall with excessive mucous contents. Atrophy of lymphoid organs was also detected, hence the bursal lesions were similar to that obtained by El-Aroussi et al. (2006) and Hameed et al. (2013). The Latter studies stated that the depletion of the lymphoid elements of birds bursa and spleen due to the toxic effect of OTA lead to various immunosuppressive effects. Thickening of intestinal mucosa could be attributed to the direct contact of OTA particles to intestinal mucosa (Kabak et al., 2009). Addition of cholestyramine or oxihumate to OTA contaminated diet led milder lesions than that of group-1, represented by only slight enlargement and mild congestion of both kidneys and liver, while other organs were grossly normal. Reduction of gross lesions in both group-2 and -3 compared with those of group-1 could be attributed to the protective effect of both cholestyramine or oxihumate on different organs of ochratoxicated chickens (Jansen-van, 2005).
Kidneys of group-1 showed degeneration or necrosis of renal tubules with replacement by extravasated RBCS beside focal leukocytic cells infiltration (Fig. 1a). Some renal tubules contained urates deposites within its lumen (Fig. 1b). Focal intertubular lymphocytic cells infiltration and interstitial fibrosis were encountered. Other renal tubules revealed massive heterophils and lymphocytes infiltration while kidneys of group-2 showed mild tubular degeneration and mild to moderate congestion of the renal tubules (Fig. 1c). Nephrotic changes in some renal tubules (Fig. 1d) were also detected in the same group in addition to a few extravasated erythrocytes among dissociated renal tubule (Fig. 1e). Kidneys of group-3 showed dilatation of some renal tubules containing casts (Fig. 1f) in some cases and others showed regeneration of some renal tubules (Fig. 1g). Marked hepatic lesions in chickens of group-1 were also observed which represented by portal fibrosis and cholestasis (Fig. 2a), in addition to mild to moderate congestion of hepatic blood vessels in some cases while. Liver of chickens in group-2 showed only moderate congestion of the hepatic blood vessels (Fig. 2b). In group-3, livers
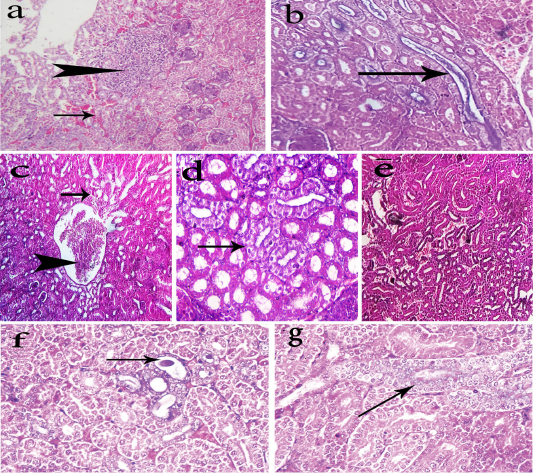
Figure 1: Photomicrographs of H&E stained kidney sections of the chickens. The kidneys of group-1 shows intertubular extravasated RBCS (arrow) with focal necrosis replaced by leukocytes cells (head arrow) (a, 200x), and urate deposition (arrow) within renal tubules (b, 400x). The kidneys of group-2 shows mild tubular degeneration (arrow) and congestion of the renal tubules (head arrow) (c, 200x), nephrotic changes in some renal tubules (arrow) (d, 400x), and few extravasated erythrocytes among dissociated renal tubule (e, 200x). The kidneys of group-3 shows dilatation of some renal tubules containing casts (arrow) (f, 400x) and regeneration of some renal tubules (arrow) (g, 400x).
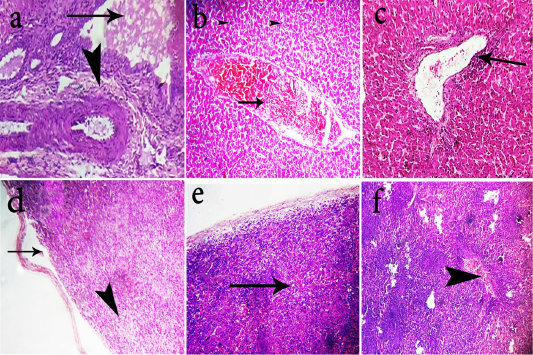
Figure 2: Photomicrographs of H&E stained liver and spleen sections of the chickens. Livers of group-1 shows portal fibrosis (head arrow) and cholestasis(arrow)(a, 400x). Livers of group-2 shows moderate congestion of the hepatic blood vessels (arrow) (b, 200x). Livers group-3 shows mild perivascular lymphocytic cells infiltration of (arrow) (c, 200x). Spleen of group-1 shows lymphoid depletion (head arrow) and splitting of capsule (arrow) (d, 400x). Spleen of group-2 shows subcapsular edema and mild depletion (arrow) (e, 200x). Spleen of group-3 shows congestion of blood vessels (arrow head) with hyperplastic white pulps (f, 200x).
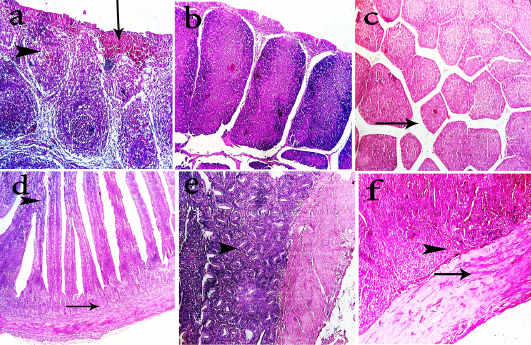
Figure 3: Photomicrographs of H&E stained bursa of fabricius and intestine sections of the chickens. Bursa of fabricius of group-1 shows mild depletion of lymphocytes (head arrow), interfollicular fibroplasia and focal hemorrhage (arrow) (a, 400x). Bursa of fabricius of group-2 shows apparently normal lymphoid follicles (b, 200x). Bursa of fabricius of group-3 shows mild interfollicular edema (arrow) (c, 200x). Intestine of group-1 shows degenerated submucosal glands (arrow) and fusion of villous tips (head arrow) (d, 400x). Intestine of group-2 shows hyperplastic intestinal glands in submucosa (head arrow) (e, 400x). Intestine of group-2 shows hyaline degeneration of muscular layer (arrow) and proliferative submucosal glands (f, 400x)
showed mild perivascular lymphocytic cells infiltration (Fig. 2c). The previous renal and hepatic lesions of group-1 were almost agreed with those obtained by (Hameed et al., 2013; Bharathi et al., 2015). The observed renal lesions may be due to the route of elimination of OTA through the kidneys and to the direct toxic action of OTA on renal parenchyma (Stoev et al., 2002). OTA inhibits protein synthesis, produces acute proximal tubular epithelial necrosis in the kidneys and inhibits normal renal uric acid secretion. The increased levels of serum uric acid and creatinine in the OTA intoxicated chickens which represented by urate deposition indicative of impaired renal function and confirms that kidney are main site of OTA toxicity (Elaroussi et al., 2008). Spleen in most cases of chicken of group-1 revealed mild to severe lymphoid depletion and splitting of splenic capsule (Fig. 2d), while spleen of group-2 showed subcapsular edema and mild depletion (Fig. 2e). Spleen of group-3 showed congestion of blood vessels with hyperplastic white pulps (Fig. 2f). Spleen lesions were almost similar to that obtained by (Stoev et al., 2004; Bharathi et al., 2015) OTA has immunotoxic properties due to its degenerative changes and cell death of immune cells because of inhibition of protein synthesis (AL-Anati and Petzinger,
Table 2: Ochratoxin-A residues in different organs of the experimental groups (mean ± SD)
| Organ /Groups | Liver | Kidney | Muscle |
| 1 | 226.93±3.21a | 214.59±3.28a | 391.41±2.89a |
| 2 | 73.02±2.54b | 89.91±2.71c | 137.25±2.24c |
| 3 | 82.42±24.95b | 102.72±3.141b | 145.82±2.39b |
Table 3: Lesions score among different examined organs of experimental groups
Affected organ of experimental chickens | Main lesions
| Lesion score in different groups (gr-1, gr-2 and gr-3) | ||
| 1 | 2 | 3 | ||
Kidney
| Hemorrhage- Degenerative changes- Coagulative necrosis- Regenerative attempts- -Urate deposition Perivascular fibrosis- | + + + ++ + + + + + + | + + + + - - | ++ + + + - - |
Liver
| Coagulative necrosis- Portal fibrosis- Portal leukocytic infiltrations- Cholestasis- -Kupffer cell proliferation | +++ ++ + ++ ++ + | + + + + + + | ++ ++ ++ + + + |
Spleen | Lymphocytic depletion- Splitting of capsule- Perivascular edema- | + + + + + + | + + + | + + + |
Bursa of Fabricius | Lymphoid depletion of lymphoid follicle- Hemorrhage- Hyperplastic lymphoid follicle degeneration- Interstitial fibrosis- Hyperplasia of epithelial covering | ++ + ++ + ++ | + -- - - + | + - + - + |
Intestine
| Submucosal leukocytic infiltration- Congestion of intestinal blood vessels- Intestinal thickenin Hyperplastic intestinal glands Enterocytes desqumation and necrosis - | ++ ++ ++ + ++ | - - + ++ + | - + + ++ + |
(-): negative; (+): mild; (++): moderate; (+++): severe
2006). Bursa of Fabricius of group-1 chickens showed mild depletion of lymphocytes with interfollicular fibroplasia and focal haemorrhage (Fig. 3a), while in group-2 revealed apparently normal lymphoid follicles (Fig. 3b), and mild interfollicular edema in group-3 (Fig. 3c). Bursa of Fabricius lesions nearly agreed with those obtained by Santin et al. (2002) and Bharathi et al. (2015). Bursal lymphoid depletion occurred reflects reduction in antibody producing cells and reduction of bursal mitotic index leading to immunosuppression (Santin et al., 2002). Intestine of goup-1 chickens revealed degenerated submucosal glands and fusion of villous tips (Fig. 3d) while intestine of group-2 chickens showed hyperplastic intestinal glands in submucosa (Fig. 3e) and intestine of group-3 showed hyaline degeneration of muscular layer and proliferative submucosal glands (Fig. 3f) In accordance, the intestinal lesions could be explained on a base of direct contact of intestinal mucosa to OTA particles (Bharathi et al., 2015). The severity of this lesion was dose and time dependent which was supported by El-Banna, (2003) who noticed catarrhal enteritis. Reduced lesions in group-3 was attributed to the effect of oxihumate on OTA toxicated chickens according to Jansen-van (2005) who stated that oxihumate had a high adsorbent capacity for OTA. Table-2 shows the residues of OTA in collected organs. The lesion scores of different examined organs of experimental groups were demonstrated in a Table 3. These results are in agreement with a recent study who exhibited a significant reduction in lesion score and aflatoxin residue in visceral organs by using antimycotoxin binders cholestyramine and oxihumate (Ali et al., 2020). Likewise another study reported the significant potential of humic acid against aflatoxicosis in broilers (Arafat and Khan, 2017).
CONCLUSION
Addition of cholestyramine and oxihumates as antimycotoxins into chicken ration had significant protective effect against OTA with superiority of cholestyramine when compared with oxihumates.
acknowledgements
The authors thank their respected institutes and university.
CONFLICT OF INTEREST
The authors declare that there is no any conflict of interest.
authors contribution
All authors except the forth one had supervision contribution .The first and fifth author had the major contributing part in paper writing, editing and reviewing in addition the role of the third, forth and fifth author in practical part implementation and finally corresponding by the fifth author.
REFERENCES






