Advances in Animal and Veterinary Sciences
Research Article
Effect of Grape Seed Oil on Hepatic Function in Adult Male Rabbits Treated with Sodium Fluoride (Part-II)
Khalisa Khadim Khudiar*, Aous Muhammad Ali Aldabaj
Department of Physiology and Pharmacology, College of Veterinary Medicine, University of Baghdad-Iraq.
Abstract | The present study was undertaken to search out the beneficial effect of natural antioxidant (Grape seed oil) against deleterious effect of fluoridation (sodium fluoride in drinking water) on hepatic function of adult male rabbits. Thirty adult male rabbits were randomly divided into three groups (C, T1 and T2) and were treated daily for 60 days as follows: Group C: Rabbits of this group were allowed to ad libitum supply of drinking water (control group), treated groups (T1 and T2) were given drinking water containing 100 ppm sodium fluoride (NaF), in addition to NaF, rabbits in group T2 were received 0.35 ml /Kg B.W of grape seed oil (GSO). Fasting blood samples were collected at different times 0, 30 and 60 days of the experiment by cardiac puncture technique for measuring the following parameters: Serum reduced glutathione (GSH), total protein (TSP) and blood glucose concentrations, as well as, serum aspartate aminotransferase (AST) and alkaline phosphatase (ALP) activities were estimated. In addition, sections from liver were taken at the end of the experiment for histopathological study. The results revealed that animals exposed to NaF (T1 group) in drinking water for 60 days showed hepatic dysfunction manifested by significant elevation in the serum AST and ALP activities with a significant decrease in serum protein and significant increase in blood glucose concentrations. A significant reduction in serum GSH concentration were also clarified comparing to T2 group. While oral intubation of grape seed oil concurrently with NaF caused a significant correction of the previous studied parameters related to hepatic functions manifested by significant decrease in serum AST and ALP activities, hypoglycemia with significant elevation in serum GSH, and TSP concentrations. Histological sections of liver in NaF treated (T1) group revealed hepatic injury manifested by mononuclear cell aggregation around the congestive blood vessel and bile duct in the buccal area together with dilatation of the sinusoid.
Keywords | Grape seed oil, Sodium fluoride, Liver enzyme, TSP, GSH, Rats
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 11, 2015; Revised | August 08, 2015; Accepted | August 09, 2015; Published | August 28, 2015
*Correspondence | Khalisa Khadim Khudiar, University of Baghdad-Iraq; Email: khuder@gmail.com
Citation | Khudiar KK, Aldabaj AMA (2015). Effect of grape seed oil on hepatic function in adult male rabbits treated with sodium fluoride (Part-II). Adv. Anim. Vet. Sci. 3(10): 550-558.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.10.550.558
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Khudiar and Aldabaj. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Fluoride is an essential trace element widely distributed in the nature. Fluoride can occur naturally in surface waters as a result of the deposition of particulates from the atmosphere and the weathering of fluoride-containing rocks and soils. Ground water can also contain high concentrations of fluoride owing to leaching from rocks. Chemical manufacturing plants and waste products can contribute fluoride to raw water sources directly through effluents or indirectly through volatilization (ATSDR, 2003). Natural geological sources and increased industriazation and attendants environmental pollution have contributed greatly to the increasing evidence of fluoride-related human health issue. High levels of fluoride in drinking water have been a potential hazard all over the world (Susheela, 2007; Susheela et al., 2013).
Chronic fluorosis is a slow and progressive process causing symptoms related to several systems, particularly musculoskeletal and bone (Srikanth et al., 2008; Xu et al., 2008; Izuora et al., 2011). Besides its hyperlipidemic effect (Khudair and Aldabaj, 2014), metabolic, functional and structural damages caused by chronic fluorosis have been reported in many tissues, including kidney (Blaszczyk et al., 2008; Inkielewicz and Czarnowski, 2008; Nabavi et al., 2013), liver (Bouaziz et al., 2006; Grucka-Mamczar et al., 2009), testis (Krasowska et al., 2004) and brain (Basha et al., 2011). Chronic fluorosis may induce oxidative stress leading to generation of free radicals and alterations in antioxidants or reactive oxygen species (ROS) scavenging enzymes (Stepniak and Czarnowski, 2010; Nabavi et al., 2012; Rupal and Narasimhacharya, 2012; Bouasla et al., 2014). This study was designed to investigate the beneficial effect of natural antioxidant against deleterious effect of sodium fluoride in drinking water in adult male rats.
MATERIALS AND METHODS
Experimantal Design
Thirty adult male rabbits were randomly divided into three groups (10 rabbit/ group) and were treated daily for 60 days as follows:
Control group: Rabbits of this group were allowed to ad libitum supply of drinking water.
Group T1: Rabbits of this group were allowed to ad libitum supply of drinking water containing 100 ppm of sodium fluoride.
Group T2: Rabbits of this group were allowed to ad libitum supply of drinking water containing 100 ppm sodium fluoride and received 0.35 ml /Kg. B.w of grape seed oil.
Fasting blood samples were collected by cardiac puncture technique at different times 0, 30 and 60 days of the experiment. Blood samples were kept in tubes, and centrifuged at 2500rpm for 15 minutes, and then serum samples were aliquoted and frozen at -20°C until analysis. Serum samples were used for measurements:Glutathione concentration (GSH) according to (Burtis and Ashood, 1999), total serum protein concentration using total serum protein kit –Linear chemicals, Barcelona, Spain), blood glucose concentration, serum aspartate aminotransferase (AST) and alkaline phosphatase (ALP) activities using blood glucose kit, ALT and AST kit (BioMeriux, Spain). Besides, sections from liver were taken at the end of the experiment for histopathological study (Luna, 1968). Statistical analysis of data was performed on the basis of Two-Way Analysis of Variance (ANOVA) using a significant level of (P<0.05). Specific group differences were determined using least significant differences (LSD) as described by (Snedecor and Cochran, 1973).
RESULTS AND DISCUSSION
Serum Reduced Glutathione (GSH) Concentration
Along the treatment period (After 30 days) a significant (P<0.05) reduction in serum GSH concentration with mean value of (17.6±0.5) was detected in NaF Treated Group (T1) comparing to the control group (21.6±0.5). Besides, the protective effect of GSO on the antioxidant status of the animal was clarified after one month of treatment, where a significant elevation (P<0.05) in serum GSH concentration (µmol/L) was detected in T2, (24.40±0.5) as compared to T1 and control groups (Table 1).
Table 1: Effect of oral administration of grape seed oil (GSO) on serum glutathione concentration (µmol/L) of rabbits treated with sodium fluoride in drinking water
|
Days |
Groups |
||
|
C |
T1 |
T2 |
|
|
Zero |
21.4±0.5 A a |
23.0±0.7 A a |
22.0±0.7 A a |
|
30 |
21.6±0.5 B a |
17.6±0.5 C b |
24.4±0.5 A b |
|
60 |
22.8±0.73 B a |
11.1±0.4 C c |
28.4±0.74 A c |
Values are expressed as mean ± SE, n = 5 each group; C: control group; T1: Animals received 100 ppm NaF in drinking water; T2: Animals received 100 ppm NaF in drinking water and 0.35 ml/Kg B.W of GSO; Capital letters denote differences between groups, P<0.05 vs. control; Small letters denote differences within group
Alterations (decrease) in the levels of reduced glutathione (GSH) and total antioxidant capacity (TAC) as well as the activities of superoxide dismutase (SOD) and catalase (CAT) were observed after NaF treatment (Inkielewicz and Czarnowski, 2008; Hanna and Mokhtar, 2009; Hassan and Abed-Aziz, 2010; Stepniak and Czarnowski, 2010). The mechanisms by which fluoride causes its deleterious effects have not been exactly determined yet. However, lipid peroxidation and oxidative stress has been suggested as one of the important mechanisms of toxic effects of fluoride (Rupal and Narasimhacharya 2011a; Grucka-Mamczar et al., 2009; Abdel-Wahab, 2013; Yamaguti et al., 2013). Taken as a whole, it can be suggested that fluoride inhibits glucose -6- phosphate dehydrogenase (G6PD) via an oxidative damage, and the subsequent decrease of Pentose phosphate pathway flow could make the cell unable to maintain the normal GSH/GSSG ratio, lowering GSH concentration and cell injury (Gazzano et al., 2005; Polimeni et al., 2008; Bergandi et al., 2010).The antioxidant protective activity of grapes was documented by many investigators (Dani et al., 2008; Abdul-Katum and Khudair, 2008; Hasseb et al., 2013; Kikalishvili et al., 2011). Reduction in MDA level and subsequent increase of endogenous antioxidant defense including GSH by active chemical constituent of grapes were also claimed (Du et al., 2007; Sano et al., 2007; Barden et al., 2008). Polyphenolic compounds present in grapes like resveratrol (Hudson et al., 2007; Alas et al., 2008; Sakla and Lorson, 2008) and procyanidins (Weber et al., 2007; Hassan et al., 2013) may be responsible for the antioxidant capability of the plant and has a protective effect against H2O2 induced oxidative damage in vitro (Houde et al., 2006).
Serum Aspartate Aminotransferase (AST) and Alkaline Phosphatase (ALP) Activities
The mean values of serum AST and ALP activities in different treated and control groups were clarified in Tables 2 and 3, respectively. A significant statistical elevation (P<0.05) in the activity of these enzymes was observed in (T1) group after 30 and 60 days of the experiment as compared to the control and other treated groups. The results also showed a significant depression (P<0.05) in serum AST and ALP activities after oral intubation of grape seed oil (T2) at day 30 of the experiment as compared to T1 and control groups. The table also showed a general trend to significant decrease (P<0.05) in the activity of these enzymes in T2 treated group compared to NaF treated group (T1) at the end of the experiment.
Table 2: Effect of oral administration of grape seed oil (GSO) on serum aspartate aminotransferase (AST) activity (IU/L) of rabbits treated with sodium fluoride in drinking water
|
Day |
Groups |
||
|
C |
T1 |
T2 |
|
|
Zero |
4.92± 0.1 A a |
5.0± 0.07A a |
4.90± 0.07A a |
|
30 |
4.94± 0.05 A a |
6.62± 0.1A b |
5.44±0.09 B b |
|
60 |
4.98± 0.08A a |
8.34± 0.15A c |
5.70±0.11B b |
Values are expressed as mean ± SE, n = 5 each group; C: control group; T1: Animals received 100 ppm NaF in drinking water; T2: Animals received 100 ppm NaF in drinking water and 0.35 ml/Kg B.W of GSO; Capital letters denote differences between groups, P<0.05 vs. control; Small letters denote differences within group
Table 3: Effect of oral administration of grape seed oil (GSO) on serum alkaline phsosphatase (ALP) activity (IU/L) of rabbits treated with sodium fluoride in drinking water
|
Days |
Groups |
||
|
C |
T1 |
T2 |
|
|
Zero |
4.70±0.18 A a |
4.94±0.09 A a |
4.58±0.31 A a |
|
30 |
4.92±0.12 C a |
8.36±0.47 A b |
5.80±0.42 B b |
|
60 |
4.72±0.22 C a |
11.94±0.39 A c |
6.14±0.24 B b |
Values are expressed as mean ± SE, n = 5 each group; C: control group; T1: Animals received 100 ppm NaF in drinking water; T2: Animals received 100 ppm NaF in drinking water and 0.35 ml/Kg B.W of GSO; Capital letters denote differences between groups, P<0.05 vs. control; Small letters denote differences within group
Elevation in transaminases (AST) and (ALP) activities was found to be related to damage in the liver and the change in hepatic functions (Eraslan et al., 2007; Kanbur et al., 2009). The increase in plasma AST and ALT activities is in agreement with the findinigs of Shanthakumari et al. (2004), Bouaziz et al. (2006) and Nabavi et al. (2012) in their studies on rats, where AST and ALT activities of rats significantly increased after treating with 10, 20, 30, 50, 100 mg/kg NaF daily for different periods (12-16 weeks), suggesting hepatic dysfunctions which were documented histologically after NaF exposure (Ersan et al., 2010). A significant increase in ALT and AST activities in fluoride toxicity was also been reported in goat (Snigh et al., 2002), cattle (Maiti and Das, 2004) and man (Shivarajashankara et al., 2001).While non-significant changes in serum ALT, AST and Total serum were observed by Xiong et al. (2007). Disturbance in antioxidant status in rat liver and impairment in its proper function has been reported after NaF consumption (Abdel-Wahab et al., 2013; Blazczyk et al., 2011; Rupal et al., 2011b; Panneerselvam et al., 2013).
Fluoride induced apoptosis and elevating lipid peroxidation - induced oxidative stress, may cause mitochondrial dysfunction and activate both hepatic caspase 9 and 3 with elevation of transaminase activity (Zhan et al., 2006). ALP activity increased after hepatic cell damage and the obstruction of bile ducts arising from cellular reproduction (Kalender et al., 2005), and its almost a consistent finding in natural and experimental fluorosis (Bouaziz et al., 2004). Changes in plasma biochemistry also revealed cytotoxic potential of excess fluoride intake to cells present in bone, liver and kidney, accordingly, elevation in ALP activity following fluoride administration may be due to lyses of osteoblasts and osteocytes (Krook and Minor, 1998) and changes in bone metabolism (Vani and Reddy, 2000). Many investigators demonstrated the efficacy of grape seed proanthocyanidins and grape juice as an inhibitor of lipid peroxidation and as a powerful free radical scavenger in vitro as well as in vivo (Dani et al., 2008; Khalifa et al., 2011; Naguib, 2011), in addition to its hepatoprotective effect (Hassan and Al-Rawi, 2013). Thus it was suggested that the active component of grapes protect the hepatocytes from liver damage and subsequent leakage of enzymes into the circulation, and may have a curative effect against elevated transaminases (Ahmed and Fatani, 2007; Al-Attar, 2015).
Total Serum Protein (TSP) concentration
Analysis of data revealed that oral intubation of GSO in combination of sodium chloride (T2) for 60 days caused significantly increased (P<0.05) in the mean value of TSP concentration comparing to T1 and control group (Table 4).
Blood proteins are important complementary constituents in the diagnosis of hepatic disease, although determination of these proteins seldom leads to specific diagnosis, but it helps to evaluate the nature, severity and progress of disease (Lumeij, 2008). Decreased protein synthesis during fluorosis was documented in rabbits (Liang et al., 2012), rats (Verma et al., 2002) and mice (Bouaziz et al., 2006). Moreover, Zang et al. (1996) reported a significant decrease in serum proteins in individuals with poor nutrition and living in high fluoride areas.
Table 4: Effect of oral administration of grape seed oil (GSO) on total serum protein (TSP) concentration g/L of rabbits treated with sodium fluoride in drinking water
|
Days |
Groups |
||
|
C |
T1 |
T2 |
|
|
Zero |
5.20±0.10 A a |
5.60±0.11 A a |
5.68±0.30 A a |
|
30 |
5.30±0.12 A a |
5.12±0.08 A b |
5.40±0.14 A a |
|
60 |
5.50±0.10 B a |
3.86±0.15 C c |
6.62±0.21 A b |
Values are expressed as mean ± SE, n = 5 each group; C: control group; T1: Animals received 100 ppm NaF in drinking water; T2: Animals received 100 ppm NaF in drinking water and 0.35 ml/Kg B.W of GSO; Capital letters denote differences between groups, P<0.05 vs. control; Small letters denote differences within group
It is evidently indicated that fluoride can disturb the metabolism of proteins and impair the activities of a series of enzymes (which are protein in nature) such as alkaline phosphatase, cholinesterase, adenylate cyclase (Zabulyte et al., 2007). This decrease in the protein synthesis may be due to impairment of peptide chain initiation (Godchaux and Atwood, 1976) or a decrease in mRNA transcription and inhibition of DNA synthesis (Jhai et al., 2002). In addition, it affects the action of Na-K-ATPase in the cell membrane which may influence the transport of amino acids into the cells (Opit et al., 1966). Moreover, it has a very strong ability to form a hydrogen bond with the phenolic hydroxyl group of tyrosine in proteins to disrupt the normal spatial conformation of various proteins (Li and Cao, 1994) and disturb protein metabolism (Birkner et al., 2000) and promote its degradation (Heidenreich et al., 1999). Finally, it can be hypothesized that fluoride-induced oxidative stress could provoke conformational changes of some key proteins that, in turn, could trigger an over expression of some stress proteins, as heat shock protein (Cullen and Sarge, 1997; Fukudo et al., 1997). These authors suggests that oxidative stress, induced by fluoride treatment, exceeded antioxidant capacity of renal cells in young mice and led to a protein denaturation and/or misfolding. It is noteworthy to mention that a case of a decrease in antioxidant status was observed in this study. Besides, NaF generates FRS down regulate the activity of enzyme important in polymerization of amino acids, has inhibitory process of elongation of peptides (Hordyjewska and Pasternaky, 2004).
Grape seed oil was found to increase the level of TSP through stimulation of protein synthesis, accelerates the regeneration process and the production of liver cells (Uma Maheswari and Rao, 2005). Beside, GSE causes inhibition of gluconeogensis and prevents catabolism of protein and its conversion to glucose (Bujanda et al., 2006), this may lead to increase level of TSP concentration in serum. The natural antioxidant present in GSO (vitamin E (60-120 mg/100g), may be responsible for its antioxidant property and for protection of DNA damage (Hassan et al., 2014) and was claimed to be the mechanism of hepatoprotective activity of GSO.
Serum Glucose Concentration
During treatment period (after 30 days) a significant (P<0.05) elevation in serum glucose concentration with mean value of (133.4±4.47) was detected in NaF treated Group (T1) comparing to NaF plus GSO T2, (112.2±6.02) and control groups (89.20±2.59).The results in (Table 5) also showed a significant (P<0.05) decrease of serum glucose concentration at the end of the experiment in GSO (124.8±1.52) as compared to T1 (196.0±7.98) group.
Table 5: Effect of oral administration of grape seed oil (GSO) on serum glucose concentration (mg/dL) of rabbits treated with sodium fluoride in drinking water
|
Days |
Groups |
||
|
C |
T1 |
T2 |
|
|
Zero |
90.20±2.13 A a |
92.6±1.50 A a |
94.0±4.03 A a |
|
30 |
89.2±2.59 C a |
133.4±4.47 A b |
112.2±6.02 B b |
|
60 |
93.2±4.61 C a |
196.0±7.98 A c |
124.8±1.52 B b |
Values are expressed as mean ± SE, n = 5 each group; C: control group; T1: Animals received 100 ppm NaF in drinking water; T2: Animals received 100 ppm NaF in drinking water and 0.35 ml/Kg B.W of GSO; Capital letters denote differences between groups, P<0.05 vs. control; Small letters denote differences within group
The effects of fluoride on glucose metabolism were examined in both in vivo and in vitro studies (Rigalli et al., 1995; Menoyo et al., 2005; Rupal and Narasimhacharya, 2011c). Inhibition of enzymes involved in cellular metabolism and alteration in boby metabolism (Rupal and Narasimhacharya, 2011a and b), is one of the mechanical toxic effects of fluoride. Enzymes involved in the glycolytic pathway, such as hexokinase, enolase, and pyruvate kinase are all subject to fluoride inhibition, may be through lowering insulin level (Gracia-Monlolvo et al., 2008). High level of fluoride were found to be associated with beta cell dysfunction and decrease sensitivity of pancreatic tissue toward insulin stimulation (Menyo et al., 2008), resulting in hypoinsulinemia and hyperglycemia. Interestingly, mitochondria may be the major target of fluoride toxicity in several organs, including the rat pancreas (Gravance et al., 2001; Dabrowska et al., 2004), where increased formation of mitochondrial O2–, decreased mitochondrial activity (Cai et al., 2007) and decreased insulin secretion were documented. There are no reports linking fluoride exposure to insulin expression or to alterations in the expression patterns of genes involved in maintaining glucose homeostasis. However, there was an evidence that fluoride exposure could influence the transcription of several genes (Zhan et al., 2006; Zhang et al., 2007; Wurtz et al., 2008). Decreased expression of insulin mRNA in pancreatic TC-6 cells could be associated with oxidative stress induced by fluoride exposure (Kaneto et al., 2005). Apoptosis plays an important role in fluoride toxicity in several cell types, including pancreatic cells (Loweth et al., 1996; Elliott, 2001), possibly because of involvement of oxidative stress which is greatly associated with a case of hyperglycemia (Robertson, 2007).
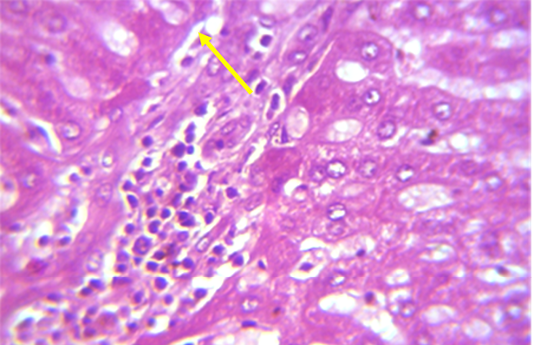
Figure 1: Section in the liver of rabbit treated with 100 ppm (NaF) for 60 days, showed mononuclear cell aggregation of blood vessels and bile duct in portal area (shown with yellow arrow) (H and E 40X)
Previous works found that Grape seed extract GSE prevents oxidative injury (Puiggros et al., 2005), modulates glucose haemostatic (Montagut et al., 2010), and has insulin mimetic effect on adiposities and adipose tissue (Pinent et al., 2004). Grape seed extract (GSE), a well-known dietary supplement, (Weber et al., 2007), with its proanthocyanidins, possess insulinomimetic properties (Decorde et al., 2009) by stimulating glucose uptake in insulin-sensitive cell lines and decreases hyperglycemia in streptozotocin (STZ)-diabetic rats (Pinent et al., 2004). It was documented that intake of GSE may be a feasible therapeutic strategy for prevention of a high-fructose diet-induced insulin resistance and oxidative stress (Suwannaphet et al., 2010). A recent study (Adisakwattana et al., 2010) has demonstrated the inhibitory activity of GSE against intestinal alpha-glucosidase and pancreatic alpha-amylase, resulting in delayed carbohydrate digestion. The hyperglycemic effect of GSO could be ascribed to its secondary metabolites (polyphenols and flavonoids). Polyphenols are known to inhibit gut glucose absorption and peripheral tissue glucose uptake by glucose transporter (Pandy and Rizvi, 2009), protect pancreatic islets cells and inhibit insulin resistance (Zunino et al., 2007; Meydani and Hasn, 2010), while flavonoids possess antidiabetogenic activity attributed to increase pancreatic secretion and insulin release from beta cells (Sharma et al., 2008; Sridhir et al., 2005).
Histological examination of Liver
A microscopic examination of sections of liver in animals treated with NaF showed mononuclear cell aggregation of blood vessels and bile duct in portal area (Figure 1). Another histological section showed granulomatus lesion consisted of aggregated macrophages and congestion of central vein and sinusoid (Figure 2). Kupffer cell proliferation was observed in liver of animals received 0.5 ml of GSO in addition to NaF (Figure 3). It is worthy to mention that histological (Structural) changes observed in Liver, supported the observed biochemical (Functional) changes in all experimental groups.
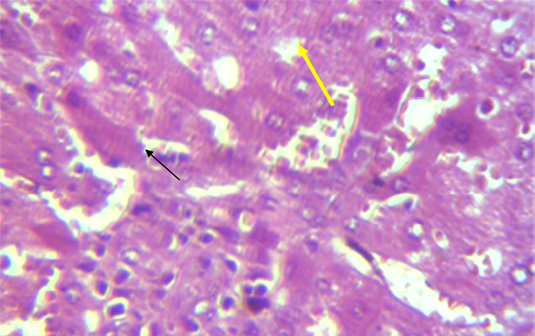
Figure 2: Section in the liver of rabbit treated with 100 ppm NaF for 60 days, showed granulomatus lesion consist of aggregated macrophages (shown with yellow arrow) and congestion of central vein and sinusoid (shown with black arrow) (H and E 40X)
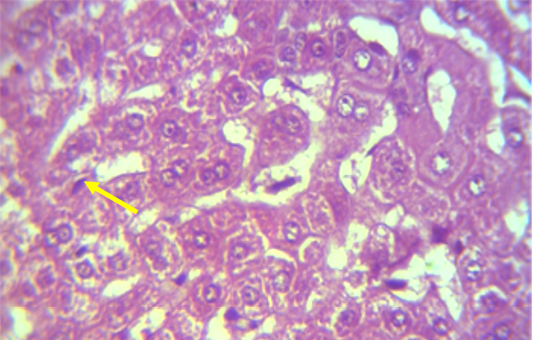
Figure 3: Section in the liver of NaF treated rabbit received grape seed oil (0.35 ml\Kg Bw) for 60 days, showed Kupffer cell proliferation (shown with yellow arrow) (H and E 40X)
CONCLUSION
GSO supplementation could alleviate hepatic changes induced in adult male rabbits by NaF as reflected by normalization of the measured liver function markers suggesting its potential hepatoprotective effect.
AKNOWLEDGEMENT
Authors of this work would like to thank Prof. Dr. Mushain AL-Zuhairy and Prof. Dr. Muhammad Jewaid Alwan for their help and support.
CONFLICT OF INTEREST
There exist not any conflict of interest.
Author’s Contribution
Khalisa Khadim Khudair, designed the experiment, gave technical support and conceptual advice and wrote the paper. Aous Muhammad Ali Al. Dabajm analyzed the data, interpreted and performed the experiment.
REFERENCES






