Advances in Animal and Veterinary Sciences
Research Article
Changes in the Biochemical Parameters of Rat Blood under the Combined Effect of Chronic Intoxication with such Heavy Metals as Copper, Zinc, Arsenic
Rumiya Tazitdinova1, Raikhan Beisenova2, Gulnur Saspugayeva2, Bakhyt Aubakirova2, Zina Nurgalieva2, Amanbek Zandybai2, Idiya Fakhrudenova1, Aigul Kurmanbayeva1
1Sh. Ualikhanov Kokshetau State University, Kazakhstan, 020000, Kokshetau, Abay Str., 76; 2L.N. Gumilyov Eurasian National University, 010000, Astana, Satpayev Str., 2, Kazakhstan.
Abstract | The accelerated growth rates of cities, industrial development and mineral extraction have led to an increase in the volume of heavy metals entering the environment. The impact of heavy metals has been studied by many scientists, but there are still limited data on the complex effect of various heavy metals. Therefore, currently, there is an increasing interest in the effect of several heavy metals on living organisms. In this research, we modeled the chronic effects of three groups of heavy metals on the body of rats, as well as determined their biochemical parameters and changes in comparison with the control group. The results of our studies have shown that when combined with chronic intoxication with heavy metal salts, the biochemical indices of blood may vary, which is caused by a violation of the functions of the liver, kidneys, and heart muscle. Against the background of the toxic effects of heavy metals, we used “Ursodex” and “Schrot Rastoropshy” as helpful drugs for the organism, which contributed to a decrease in the toxicity of metals. The level of the negative effect of these elements on the biochemical indices of the blood of experimental animals decreased.
Keywords | Heavy metals, Intoxication, Rat blood, Blood counts, Biochemical analysis, Biochemical indices
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 30, 2018; Accepted | August 31, 2018; Published | October 06, 2018
*Correspondence | Rumiya Tazitdinova, Sh. Ualikhanov Kokshetau State University, Kazakhstan, 020000, Kokshetau, Abay Str., 76 Email: irm85@mail.ru
Citation | Tazitdinova R, Beisenova R, Saspugayeva G, Aubakirova B, Nurgalieva Z, Zandybai A, Fakhrudenova I, Kurmanbayeva A (2018). Changes in the biochemical parameters of rat blood under the combined effect of chronic intoxication with such heavy metals as copper, zinc, arsenic. Adv. Anim. Vet. Sci. 6(11): 492-498.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.11.492.498
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Tazitdinova et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Recently, there has been a sharp increase in the volume of accumulation of pollutants in the environment, the composition and properties of which are very diverse. In the gold mining and processing areas as well as in the chemical industry, the main pollutants are heavy metals. In agricultural production, soil contamination with heavy metals is associated with the use of fertilizers and pesticides (Granero and Domingo, 2002; Cao et al., 2010; Tongesayi et al., 2013).
Heavy metals are found everywhere as a result of natural processes and anthropogenic influences. The level of natural heavy metals is relatively low. The concentration of heavy metals increases due to anthropogenic influences such as mining, irrigation, pesticide use and chemical fertilizers (Granero and Domingo, 2002; Cao et al., 2010; Tongesayi et al., 2013). Environmental contamination can also occur due to metal corrosion, atmospheric deposition, metal ion erosion and heavy metal leaching, as well as the re-suspension of sediment and the evaporation of metals from water resources into soil and groundwater (Nriagu, 1989). In addition, the intensive construction of urban and rural infrastructure, the increase in the number of vehicles and general lifestyle changes, especially in developing countries, exacerbated the problem by increasing the point sources of metal emissions. Because of their prevalence, toxicity and persistence, heavy metals are of particular concern in any ecological system. The real problem is that the accumulation of heavy metals in urban soils, sediments, road dust and water bodies should be considered as chemical pollutants (Kumar et al., 2017; Bhattacharjee and Goswami, 2018; Kenston et al., 2018; Rehman et al., 2018).
Heavy metals are defined as metal elements that have a relatively high density in comparison with water (Fergusson, 1990). Given that gravity and toxicity are interrelated, heavy metals also include metalloids, such as arsenic, that can cause toxicity at low levels of exposure (Duffus, 2002; Singh et al., 2015).
It has been reported that certain metals, such as Co, Cu, Cr, Fe, Mg, Mn, Mo, Ni, Se and Zn, are the essential substances involved in various biochemical and physiological processes. They are part of some enzymes that participate in the oxidation-reduction reactions of the body (World Health Organization, 1996). However, at higher concentrations, they can lead to poisoning. The oral entry of such metals as Pb, Cd, Hg, As, Ba and Cr may pose a great threat to human health, as they accumulate in living organisms and remain there until they are metabolized and isolated (Smith et al., 2012).
Heavy metals are transmitted through the food chain, accumulating in the soil, plants, and therefore are very dangerous to humans and their health. They are the most dangerous pollutants of the environment, possessing a wide range of effects, leading to the irreversible pathologies of limbs, tissues and systems in the body (Mitsiev, 2015). They have toxic, allergic and carcinogenic effects on the entire organism. Depending on the dose and the path of intoxication, some heavy metals can selectively accumulate in a particular organ or tissue, disrupting their integrity and function (Miller, 2002).
In biological systems, heavy metals have an influence on cellular organelles, as well as on the cell membrane, mitochondria, lysosomes, endoplasmic reticulum, nucleus and some enzymes involved in metabolism and detoxification (Wang and Shi, 2001). As a result of the reaction of the organism, the state of free radical oxidation is changed and the control over the process of lipoperoxidation is lost, which increases the permeability of biomembranes (Shukla and Chandra, 1987).
Pollutants containing more than one metal can cause a different effect, in contrast to the effect of only one metal. It is known that the combined effect of Cd and As after intraperitoneal administration was more toxic than that of only one metal. People are often exposed to a combination of metals in everyday life and in the workplace. The toxic effect of the chronic doses of such mixtures has not been sufficiently studied (Goyer, 2014). There is insufficient information on the long-term combined effects of environmental pollutants.
Currently, most studies focus on the combined effect of two or three metals, because people are mostly under the toxic combined effect (Haiyan et al., 2014).In the studies of Khanturina GR (2012) on laboratory rats, the toxic effect of heavy metal salts on the functional systems of the body is shown by the results of hematological and biochemical blood indices, the transcapillary exchange of proteins, and the resistance of erythrocyte membranes. The genotoxic effect is discussed in a comparative aspect. In heavy metal intoxication, the liver and kidneys are primarily affected, as it is through them that these substances are excreted from the body (Khanturina and Khanturin, 2006).
MATERIALS AND METHODS
Animals
The experiments were performed on 100 white, outbred, mature rats. The weight of each rat was about 220-250 gr. The common rules of use and normal conditions for animals were kept. The animals were put to sleep using anesthesia with ether, then their blood was taken for biochemical studies. The chronic intoxication of rats was carried out within three months.
Experimental animals were divided into 10 groups: The first control group (n = 10) consisted of experimental animals, which were kept on a standard laboratory diet. The second group (n = 10) consisted of animals that received the intragastric solutions of zinc sulfate and copper sulfate salts. The third group (n = 10) consisted of animals that received the solutions of copper sulfate and sodium arsenite salts. The fourth group (n = 10) consisted of animals, which were injected with the solutions of zinc sulfate and sodium arsenite salts. The fifth group (n = 10) consisted of animals that received the solutions of zinc sulfate and copper sulfate salts, as well as “Ursodex”. The sixth group (n = 10) consisted of animals that received the solutions of copper sulfate and sodium arsenite salts, as well as “Ursodex”. The seventh group (n = 10) consisted of animals, which were injected with the solutions of zinc sulfate and copper sulfate salts, as well as “Schrot Rastoropshy”. The eighth group (n = 10) consisted of animals, which were injected with the solutions of copper sulfate and sodium arsenite salts, as well as “Schrot Rastoropshy”. The ninth group (n = 10) consisted of animals, which were injected with the solutions of zinc sulfate and sodium arsenite salts, as well as “Ursodex”. The tenth group (n = 10) consisted of animals, which were injected with the solutions of zinc sulfate and sodium arsenite salts, as well as “Schrot Rastoropshy”.
Preparation of Solutions
During chronic intoxication (within 90 days), the aqueous solutions of heavy metal salts were used. Their preparation was based on LD50 dosage, according to 1/10 part
Table 1: Biochemical indices of rat blood after chronic intoxication with zinc, copper and arsenic salts.
| Blood indices | Groups of experimental animals | |||||||||
| Control group | Second group | Third group | Fourth group | Fifth group | Sixth group | Seventh group | Eighth group | Ninth group | Tenth group | |
| ALT, ukat/L |
1,99 ±0,08 |
1,30 ±0,08* |
0,74 ±0,02* |
1,19 ±0,01* |
0,85 ±0,009* |
1,23 ±0,15* |
2,15 ±0,07* |
0,93 ±0,006* |
0,73 ±0,005* |
1,1 ±0,10* |
| AST, ukat/L |
3,2 ±0,12 |
5,0 ±0,12* |
3,5 ±0,09** |
4,66 ±0,06* |
2,7 ±0,02* |
5,96 ±0,028* |
4,15 ±0,022* |
3,5 ±0,02* |
4,15 ±0,04* |
4,28 ±0,36** |
| Glucose, mmol/L |
4,56 ±0,49 |
2,25 ±0,18* |
1,80 ±0,04* |
2,16 ±0,22* |
1,26 ±0,20* |
1,99 ±0,38** |
2,75 ±0,06* |
1,78 ± 0,06* |
0,93 ±0,017* |
1,43 ±0,08* |
| Total bilirubin, umol/L |
1,5 ±0,12 |
2,25 ±0,18** |
2,5 ±0,19* |
2,03 ±0,08* |
2,65 ±0,11* |
2,77 ±0,23* |
5,1 ±0,17* |
4,85 ±0,20* |
2,6 ±0,02* |
1,69 ±0,02* |
| Creatinine, mmol/L |
48,55 ±0,59 |
51,0 ±0,47* |
44,4 ±0,22* |
37,2 ±1,76** |
53 ±2,36* |
37,9 ±1,87** |
50,5 ±0,59* |
50,0 ±0,24* |
48,0 ±0,24* |
36,5 ±0,59* |
| Urea, mmol/L |
7,55 ±0,11 |
3,4 ±0,50* |
1,83 ±0,10* |
1,45 ±0,05* |
1,8 ±0,19* |
3,78 ±0,67* |
1,5 ±0,12* |
3,25 ±0,06* |
1,78 ±0,13* |
5,06 ±0,50* |
Note: * - differences are significant compared to the control group of animals at p <0.05; n is the number of animals in groups.
of LD50. During chronic combined intoxication, the daily dose of copper sulfate II was 52mg/kg, zinc sulfate – 70 mg/kg, sodium arsenite – 4.1 mg/kg. The solutions were administered intragastrically with the help of a special probe, which consisted of a tip placed on a syringe.
Treatment of Poisoned Rats
After chronic intoxication (within 45 days) with the solutions of heavy metal salts corresponding to the groups of rats, the intracastal solutions of “Ursodex” and “Schrot Rastoropshy” were injected. Three groups of rats received “Ursodex”, three other groups – “Schrot Rastoropshy”. The daily dose of “Ursodex” was14 mg/kg, “Schrot Rastoropshy” –24 mg/kg.
Blood Sampling for Research
Blood for biochemical studies was selected from the carotid artery (8 ml), arterial blood was collected in a heparinized tube. After settling, blood clotting was observed. Blood was centrifuged for 15 minutes at 3,000 rpm.The resulting serum was transferred to secondary tubes, which were then placed in an analyzer to determine the content of alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin, glucose, creatinine, and urea. The biochemical analysis of blood was performed on Photometer 50/10, using the following methods: the activity of alanine aminotransferase and aspartate aminotransferase was determined by the Reitman-Frenkel method, glucose – by the glucose oxidase method, urea – by the unified method for color reaction with diacetylmonoxime, and creatinine – by the Jaffe method with deproteinization.
Statistics
The processing of the results was carried out using descriptive and parametric statistics on a personal computer using Microsoft Office Excel software and Statistics for Windows. The mean (M) and the standard error of the arithmetic mean (m) were calculated. The significance of differences in the arithmetic mean was estimated using Student’s t-test (t) and significance level (p). Differences were considered statistically significant at p <0.05.
RESULTS AND DISCUSSION
In this study, changes in the biochemical indices of rat blood were studied as a result of chronic intoxication with the solutions of copper sulfate, zinc sulfate and sodium arsenite (see Table 1).
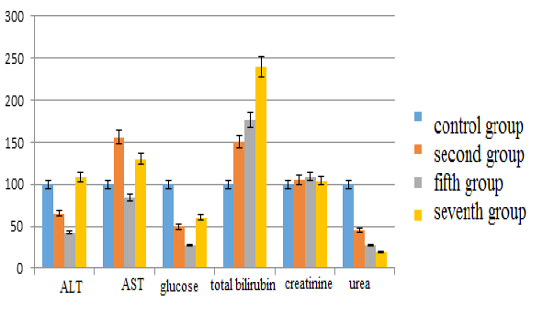
The main results of the study are as follows: in the second group the content of alanine aminotransferase decreased by 34.67% (p <0.05), in the fifth group corrected with”Ursodex” – by 57.29% (p <0.05), while in the seventh group corrected with “Schrot Rastoropshy” its content increased slightly (8%) (p <0.05) compared with the control group. Aspartate aminotransferase levels in the second group increased by 56.25% (p <0.05), in the fifth group – decreased by 15.63% (p <0.05) and in the seventh group – increased by 29.69% (p <0, 05). The glucose level in the second group decreased by 50.66% (p <0.05), in the fifth group – by 72.37% (p <0.05), in the seventh group – by 39.69% (p <0.05). The content of total bilirubin in the second group increased by 50% (p <0.05), in the fifth group – by 76.67% (p <0.05), in the seventh group – by 140% (p <0.05). The creatinine content in the second group increased insignificantly by 5.05% (p <0.05), in the fifth group – by 9.17% (p <0.05), in the seventh group – by 4% (p <0.05). The urea indices in the second group decreased by 54.97% (p <0.05), in the fifth group – by 72.45% (p <0.05), in the seventh group – by 80.13% (p <0.05) (see Figure 1).
The parameters of alanine aminotransferase compared with the control group in the third group decreased by 62.81% (p <0.05), in the sixth group – by 38.19% (p <0.05), in the eighth group – by 53.27% (p <0.05). The content of aspartate aminotransferase in the third group increased by 9.38% (p <0.05), in the sixth group – by 86.25% (p <0.05), in the eighth group – by 9.38% (p <0.05). The glucose content in the third group decreased by 60.53% (p <0.05), in the sixth group – by 56.36% (p <0.05), in the eighth group – by 14.10% (p <0.05). The bilirubin indices increased in the third group by 66.67% (p <0.05), in the sixth group – by 84.67% (p <0.05), in the eighth group – by 233.33% (p <0.05). The creatinine content decreased in the third group by 8.5% (p <0.05), in the sixth group – by 21.94% (p <0.05), and in the eighth group it increased by 2.99% (p <0.05). The urea content in the third group decreased by 75.76% (p <0.05), in the sixth group – by 49.90% (p <0.05), and in the eighth group – by 56.95% (p <0.05) (see Figure 2).
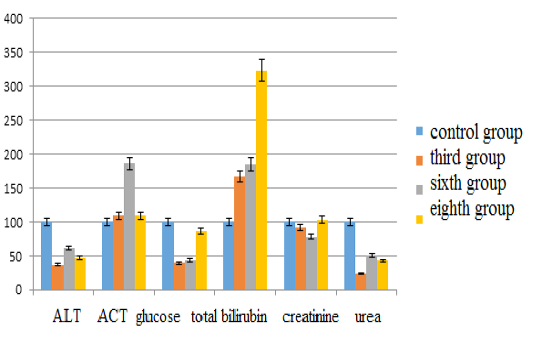
Figure 2: Changes in biochemical blood indices during chronic intoxication with the solutions of copper sulfate and sodium arsenite against the background correction with Ursodex and Schrot Rastoropshy preparations
The activity of alanine aminotransferase in comparison with the control group in the fourth group decreased by 40.20% (p <0.05), in the ninth group –by 63.32% (p <0.05), in the tenth group –by 44.72% (p <0.05). Aspartate aminotransferase levels in the fourth group increased by 45.63% (p <0.05), in the ninth group –by 29.69% (p <0.05), in the tenth group –by 33.75% (p <0.05). The glucose content in the fourth group decreased by 52.63% (p <0.05), in the ninth group –by 79.61% (p <0.05), in the tenth group– by 68.64% (p <0.05). The bilirubin content increased in the fourth group by 35.33% (p <0.05), in the ninth group –by 73.33% (p <0.05), in the tenth group –by 12.67% (p <0.05). Creatinine values decreased in the fourth group by 23.38% (p <0.05), in the ninth group –by 1.13% (p <0.05), in the tenth group –by 24.82% (p <0.05). The urea content in the fourth group decreased by 80.79% (p <0.05), in the ninth group –by 76.42% (p <0.05), and in the tenth group– by 32.98% (p <0.05) (see Figure 3).
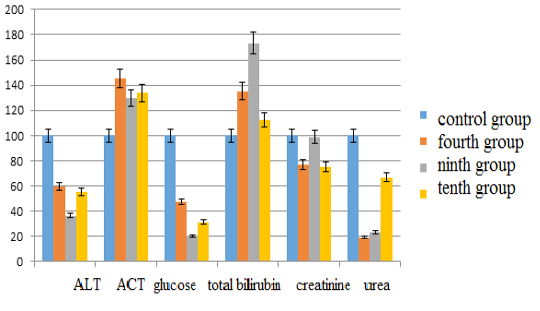
Figure 3: Changes in biochemical blood indices during chronic intoxication with the solutions of zinc sulfate and sodium arsenite salts against the background correction with Ursodex and Schrot Rastoropshy preparations
During the experiment it was revealed that the content of alanine aminotransferase decreases in the second, fifth, third, sixth, eighth, fourth, ninth and tenth groups. The greatest decrease in the alanine aminotransferase index was observed in the third and ninth group. The decrease in enzyme concentrations is probably associated with severe liver diseases – necrosis or cirrhosis, caused by a significant reduction in the number of active cells that synthesize it. It can also be associated with the presence of malignant neoplasms in the body, violations of liver function, diseases of the digestive system, as well as damages to cell membranes. Consequently, we can assume the most damaging is the effect of liver cells, due to the combined effects of the solutions of copper sulfate and sodium arsenate, zinc sulfate and sodium arsenate. In the seventh group, against the background correction with “Schrot Rastoropshy”, the level of alanine aminotransferase remained within the norm. Apparently, this contributed to the content of flavonoid silymarin in this preparation, the regeneration of liver cells, as well as the lowest toxicity of this group of metals.
In the research groups there was an increase in the level of aspartate aminotransferase. Only in the fifth group, in the context of intoxication with the solution of zinc sulfate and copper sulfate against the background correction with Ursodex, this indicator approached the norm. An increase in the content of aspartate aminotransferase may be indicative of inflammatory processes in the liver and a violation of the cardiac muscle function. In addition, the high value of transaminase ACT can talk about extensive or multiple tumors. The de Ritis coefficient, which shows the amount of AST and ALT relative to each other, is significantly greater than 2, which indicates serious damage to the heart muscle and the development of cardiovascular diseases. However, in the seventh group this indicator reached the norm. Apparently this contributed to the protective property of the drug “Schrot Rastoropshy”.
It is known that insulin causes a sharp rise in the level of insulin, which “absorbs” glucose in blood and causes a decrease in its level (Genes, 1970; Balabolkin, 2002; Dedov and Fadeev, 1998). The pronounced chronic hypoglycemia may be associated with the damage to the brain and nerve cells and cause the energy starvation of the cells. It is known that a lack of insulin leads to a disruption in the availability of basic substrates for plastic and energy metabolism and the activity of intracellular enzymes. The activation of oxidative stress associated with hyperglycemia results in damage to enzyme systems, namely carbohydrate and nitrogen metabolism (Brownlee, 2001). An increase in the metabolic load on the liver with hypoglycemia can have an adverse effect on metabolism in the liver and intestines (Balabolkin, 2002; Dedov and Fadeev, 1998). The results of the study showed a decrease in blood glucose – hypoglycemia, in all experimental groups, which is probably due to liver and kidney pathology, violations of the synthesis of thyroid hormones, violations of the adrenal glands, and new beta cells of the pancreas that are capable of producing insulin – insulinoma.
In the context of our experiment, a significant increase in the total content of bilirubin in blood (hyper bilirubinemia) was observed in all experimental groups. This may be due to the increase in the hemolysis of erythrocytes as a result of the toxic effect of heavy metals on blood cells. The increased disintegration of red blood cells probably leads to the enzymatic cleavage of more hemoglobin in the cells of the reticuloendothelial system. As a result, a large amount of indirect bilirubin is formed, which is released into the bloodstream.An increase in the level of bilirubin in blood can indicate such violations of the liver cells as an increase in the permeability of the outer membrane or its complete destruction, and as a consequence, the release of the contents of hepatocytes into the blood. It can also indicate a slowing of the motor activity of the biliary tract (dyskinesia), which enhancesthe bile pressure inside the bile capillaries, their stretching, increasing the permeability of the bile duct walls, and the penetration of bile contents into the blood. These changes are probably related to the toxic effects of the solutions of zinc sulfate salts, copper sulfate and sodium arsenite.
In the study groups, minor changes in the content of creatinine in blood were within normal limits. However, there was a decrease in urea concentrations in all experimental groups. This can be associated with severe liver damage, as urea (residual nitrogen) is formed mainly in the liver. Urea is known to be excreted from the body with urine, therefore its content in blood speaks about the function of the kidneys. But since the concentration of creatinine is within normal limits, and it is excreted from the body only by the kidneys, then probably this cannot indicate any violation of kidney function.
CONCLUSION
The conducted study presents a special approach to the analysis of the combined toxic effect associated with exposure to heavy metals. Our goal was to determine the changes in biochemical blood parameters during chronic intoxication and establish the relationship between them.
Thus, our study showed that chronic intoxication with zinc, copper and arsenic salts causes changes in the liver parenchyma and violates the antitoxic, excretory, glycogen-forming deaminizing functions of the liver.The reduction in the level of alanine aminotransferase is probably associated with the decrease in active liver cells and the lack of vitamin B6. The increase in the aspartate aminotransferase index may indicate a disruption in the integrity of cell membranes. Aspartate aminotransferase is part of the cell membrane structure. Hypoglycemia is probably due to excess insulin in the blood circulation, which reduces the formation of glucogenic amino acids, mainly in skeletal muscles, as well as the suppression of glycogenolysis and gluconeogenesis in the liver. The strongly expressed bilirubinemia seems to be a manifestation of toxic liver damage. The decrease of urea in blood serum is presumably associated with damage to liver cells, as a result of which the synthesis of urea in the liver is disrupted, which leads to an increase in the concentration of ammonia in blood and the development of hepatic encephalopathy.
When “Ursodex” was used, the level of aspartate aminotransferase approached the control values, while in the case of “Schrot Rastoropshy”, the parameters of alanine aminotransferase, aspartate aminotransferase, creatinine and glucose in the seventh and eighth groups reached the values of the control data, which testifies to the favorable effect of the drug on the liver. Our results are consistent with the studies of Khanturina GR (2012) showing that a positive effect is achieved through the stabilization of cell membranes and lysosomes, the neutralization of toxic free radicals and the anti-inflammatory action by binding of enzyme proteins in the intercellular space. As known, Rastoropshy contains flavonoid silymarin, which helps to restore the cellular membranes of hepatocytes, and the powder of milk thistle is a good sorbent that removes toxicants from the body.
Since our study uses an experimental model of animals, its results can be interpreted with respect to humans, and therefore they are very important. They help understand the mechanism of the effect of chronic doses of heavy metals on biological systems. The long-term combined effects of metals are common throughout the world and cause various diseases. Therefore, it is extremely important to continue and expand research in this direction. The positive effect of the drug “Schrot Rastoropshy” is of great importance in the treatment of liver diseases.
ACKNOWLEDGEMENTS
There is no financial support for this research.
CONFLICT OF INTEREST
We declare that we have no conflict of interest.
AUTHORS CONTRIBUTION
R. Tazitdinova, R. Beisenova, G. Saspugayeva, B. Aubakirova, Z. Nurgalieva, A. Zandybai, I. Fakhrudenova and A. Kurmanbayeva conducted the research, while all the authors participated in writing and proof reading of the manuscript.
REFERENCES