South Asian Journal of Life Sciences
Research Article
Biology and Food Utilization Efficacy of the Small Grass Yellow Eurema brigitta (Cramer) (Lepidoptera: Rhopalocera: Pieridae) in the Eastern Ghats of Southern Andhra Pradesh
Harinath Palem1, Suryanarayana Kanike1, M. Venkata Reddy2, Venkata Ramana Sri Purushottam1*
1Department of Zoology, Yogi Vemana University, Y.S.R Kadapa District- 516 003, India; 2Department of Zoology, Sri Krishnadevaraya University, Ananthapuram - 515003, Andhra Pradesh, India.
Abstract | We describe the life history of the Small Grass Yellow Eurema brigitta (Cramer), monthly occurrence and seasonality of early stages, larval performance in terms of food consumption and utilization, and the wide length of the life cycle. Our study was conducted during 2014-15 at Yogi Vemana University botanical garden, Kadapa (78o 42’44.539o E, 14o 28’25.59 N), South India. Field study indicated that a small grass yellow was in continuous flight and reproduction, with highest densities of early and adult stages occurring during September-November representing the most favorable period, the time of the entire South-West monsoon. The study reputes that the Pierid butterfly “Eurema brigitta” would be with wings and breed throughout the year. The polyphagous larvae utilize several legumes as hosts including Cassia mimosoides on which the larvae were reared and studied. The life history completed in just 20-27 days, with egg lasting 3-4, larvae 11- 16 and pupae 6-7 days under laboratory conditions (27°C temperature). The Short life cycle history permitted 11-12 broods yearly, making the butterfly multivoltine. Total larval development time includes length, weight, growth, and nutritional parameters like consumption index (CI), Growth Rate (GR), Approximate Digestibility (AD), Efficiency of conversion of ingested food to body substance (ECI) and Efficiency of conversion of Digested food to body substances (ECD). Among the five instars obtained, the last one accounted for about 73.7% of total weight gain for food consumption of 66.4% of the entire larval period. The values of CI, GR, and AD across the instars decreased as the larvae aged. The average values of CI and GR are 0.59 and 7.71 respectively and that of AD is 91.4%. In contrast the values of ECD and ECI increased as the larvae aged; their respective average values are 14.2% and 11.96%. The adult foraged on 5 floral species.
Keywords | Eurema brigitta, Life history, Mudpuddling, Food consumption, Eastern Ghats, Andhra Pradesh
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | February 09, 2015; Accepted | December 10, 2015; Published | December 26, 2015
*Correspondence | Dr. S. P. Venkata Ramana, Assistant Professor, Department of Zoology, Yogi Vemana University, Vemanapuram, Kadapa, Y.S.R Kadapa district- 516 003, Andhra Pradesh, India; Email: akshay@yogivemanauniversity.ac.in; haributterfly.yvu@gmail.com
Citation | Palem H, Kanike S, Reddy MV, Purushottam VRS (2015). Biology and food utilization efficacy of the small grass yellow Eurema brigitta (Cramer) (Lepidoptera: Rhopalocera: Pieridae) in the Eastern Ghats of Southern Andhra Pradesh. S. Asian J. Life Sci. 3(2): 63-71.
DOI | http://dx.doi.org/10.14737/journal.sajls/2015/3.2.63.71
ISSN | 2311–0589
Copyright © 2015 Palem et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Butterflies have been studied systematically since the early 18th century and about 19,238 species are documented worldwide (Heppner, 1998; Kunte, 2000). Shields (1989) had given a world total of 17,280 species, and Gaonkar (1996) mentioned 16,823 world species.
Of the estimated 20,000 – 30,000 species of butterflies occurring globally, nearly 1501 species are known to occur in India. Several field guides for the identification of the Indian butterflies are available (Wynter – Blyth, 1957; Haribal, 1992; Gay et al., 1992; Evans, 1932; Gunathilagaraj et al., 1998; Kunte, 2000). A list of the works giving the descriptions of the life histories was given by Pant and Chatterjee (1950), of which those of Bell (1909 – 1927) are important.
Among the insects, butterflies are an interesting group which have greater aesthetic value that makes them very
Figure 1: Conservation Status of Eurema brigitta
attractive to humans. They are also a very important unit of any ecosystem due to their inter–relationship with plant diversity. Butterflies were thought to be efficient for plant pollination as they visit different flowers for nectar, but this is debatable and minor (Subba and Meera, 1984, 1986; Ramana, 2010), when compared with functions of major pollinator groups like bees and true flies. Butterflies are, however, good indicators of environmental change as they are directly affected by habitat modification and loss, atmospheric temperature and weather conditions (Kunte, 2000; Tiple, 2006; Harinath et al., 2014, 2015). Haribal (1992) noted that the life histories of nearly 70% of the Indian species require description. Hence, we began studies to address the situation. Here we describe the life history of Eurema brigitta.

Figure 2: Classification of Eurema brigitta
E. brigitta known as the Broad-bordered Grass Yellow, Small Grass Yellow, or No Brand Grass Yellow (Figure 2) was a small (adult wingspan 30-40 mm), bright yellow butterfly in family Pieridae. There are about 55 species in genus Eurema; E. brigitta can be distinguished from its similar congenitors by the lack of black spots on the underside of the forewing. One of the 15 most common butterflies in Africa and Southeast Asia is the Small Grass Yellow which is widespread in almost all countries of both continents, including Arabia, Madagascar, and other islands. Here we describe the life history of small Brand Grass Yellow Eurema brigitta because of its reproductive efficiency that depends on lifestyle and feeding pattern (Muthukrishnan and Pandian, 1987; Ramana et al., 2001; Kumar et al., 2012, Harinath et al., 2014). We also studied larval performance with respect to food utilization by feeding them on a daily supply of pieces of fresh leaf of the Cassia plant.
Subspecies: (The Small Grass Yellow (Eurema brigitta (Cramer)) Encyclopedia of LIFE)
- Eurema brigitta hainana (Moore) (Hainan)
- Eurema brigitta rubella (Wallace) (Sri Lanka, India, Burma to southern China, Nicobars)
- Eurema brigitta formosana (Matsumura) (Taiwan)
- Eurema brigitta yunnana (Mell)
- Eurema brigitta australis (Wallace) (Australia, New Guinea, Papua New Guinea)
- Eurema brigitta brigitta the broad bordered grass yellow (Tropical Africa)
- Eurema brigitta pulchella (Boisduval) (Madagascar, Mauritius, Comoro Islands, Aldabra Islands)
- Eurema brigitta drona (Hors field) (Sumatra, Java to Lombok)
- Eurema brigitta senna (C. & R. Felder) (Peninsular Malaya, Singapore, Indochina)
- Eurema brigitta fruhstorferi (Moore) (eastern Indo-China)
- Eurema brigitta ina Eliot (southern Sulawesi)
MATERIAL AND METHODS
During the major study on the biology and ecology of South Indian butterflies, the small grass yellow butterfly Eurema brigitta (Moore) of Pieridae was found ovipositing on several leguminous plants including Cassia tora, Cassia occidentalis, Cassia siamea, Mimosa pudica, Samanea saman and Peltophrum. The eggs laid on Cassia mimosoides, Cassia tora were used for the present study. The host plants Cassia mimosoides distributed at the Yogi Vemana University campus botanical garden, Kadapa (78 42’ 44.539 E 14 28’ 24.59 N) (Figure 3) were observed for enumerating the eggs, larvae, pupae once every month. Freshly laid eggs along with the substrate leaf material were collected in Petri dishes of 4 and 9 cm diameter, brought to the laboratory at the Yogi Vemana University and incubated at around 27˚C.
The emerged larvae were transferred onto tender fresh leaves of Cassia mimosoides and were followed until pupation. The number of instars as indicated by skin shedding
Table 1: Diural activity pattern of Eurema brigitta on the floral species (Visits in each hour given as percentage of total daily activity)
|
Name of the species |
Timetable |
|||||||||||
|
6-7 |
7-8 |
8-9 |
9-10 |
10-11 |
11-12 |
12-13 |
13-14 |
14-15 |
15-16 |
16-17 |
17-18 |
|
|
Sida acuta burm |
0 |
0 |
17-19 |
23-29 |
11-14 |
0 |
16-21 |
18-23 |
0 |
0 |
6-11 |
0 |
|
Tridax procumbens |
0 |
0-0 |
0-0 |
0-0 |
10-11 |
15-20 |
21-25 |
0 |
0 |
0 |
0 |
0 |
|
Nerium odorum |
0 |
24-31 |
28-33 |
0 |
13-21 |
21-24 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Catharanthus roseus |
0 |
0 |
0 |
0 |
0 |
12-16 |
14-22 |
35-39 |
1-5 |
14-20 |
0 |
0 |
|
Ixora arborea,Roxb.ex sm. |
0 |
0 |
0 |
6-11 |
13-21 |
18-25 |
16-20 |
11-15 |
6-10 |
1-4 |
1-4 |
1-5 |
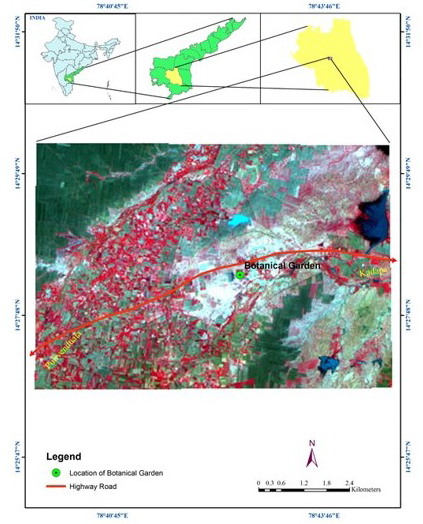
Figure 3: >Map showing Study Area
were noted. Particulars of each instar including food consumption and weight gain were recorded. Using the formulae of Waldbauer, larvae performance was assessed in terms of growth rate (GR), Consumption index (CI), Approximate digestibility (AD), Efficiency of conversation of ingested food to body tissue (ECI), and Efficiency of conversation of digested food into body tissues (ECD). The relation of food consumption and growth of larvae was analyzed statistically using Karl Pearson’s regression and correlation. Pre-pupal behavior and pupal characters were recorded. Adult nectar resources, their blooming periods, corolla lengths, particulars of nectar, butterfly proboscis length, foraging speed and diurnal activity were recorded. The growing larvae were observed regularly to note the change of instar, and characters including length, width and weight measurements. As the larvae grew, they needed more space and hence increased space was provided by transferring the growing larvae to bigger Petri dishes
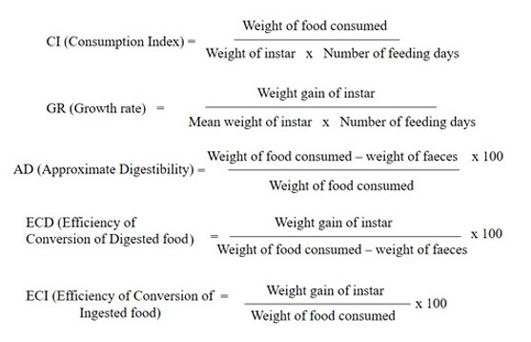
Figure 4: Waldbauer (1986) formula
(15 cm × 2.5 cm depth). Larval performance in terms of food utilization indices was calculated as described by the formulae given by Waldbauer (1968) (Figure 4).
RESULTS AND DISCUSSION
Description
Adult description and nectar resources: Adult wingspan was 20-25 mm. The male was bright yellow with upper forewing apex and terminal broader. The female also bears broader black border on the forewings. The underside of the forewing of both male and female individuals feed on floral nectar resource (Table 1).
Mud-puddling behavior: Adults of many families of Lepidoptera feed from puddles carrion and exacta (Norris 1936; Downes 1993, Adler 1982) (Figure 5). Such behavior was termed “puddling” and many involve aggregations of individuals feeding at a location which was used repeatedly. Sodium which may be on another side, a scarce nutrient in the adult diet triggers puddling behavior at least in Papilio and Eurema species.
Habitats: The common grass yellow was found all over India and was abundant in many places. It occurs in large open patches in the evergreen, semi-evergreen and deciduous forests and in scrub and grasslands near human habitations in urban and rural areas. It was among most common butterflies. It was widespread species and occurs mostly in the tropical and subtropical areas of Asia, Africa and all over India. Although it occurs round the year, it is most common in the post monsoon months. However, in certain areas such as gardens and habitations, it may be seen in the considerable numbers throughout the year owing to the variety of water and light regions in these habitats and availability of larval host plants and adult feeding plants all the months. It has weak fluttering flight. It feeds mostly on the small low growing flowers. It was also a regular member at the mud puddling assemblages.
Larval host plants: The common grass yellow was among the stages. Its hosts are leguminous plants mostly belonging to the families – Mimosaceae (acacias, touch-me-not and their relatives), Caesalpiniaceae (Cassia mimosoides) and Fabaceae. New host plants are constantly being added to the list of known host plants and it seems as the list will continue to grow.
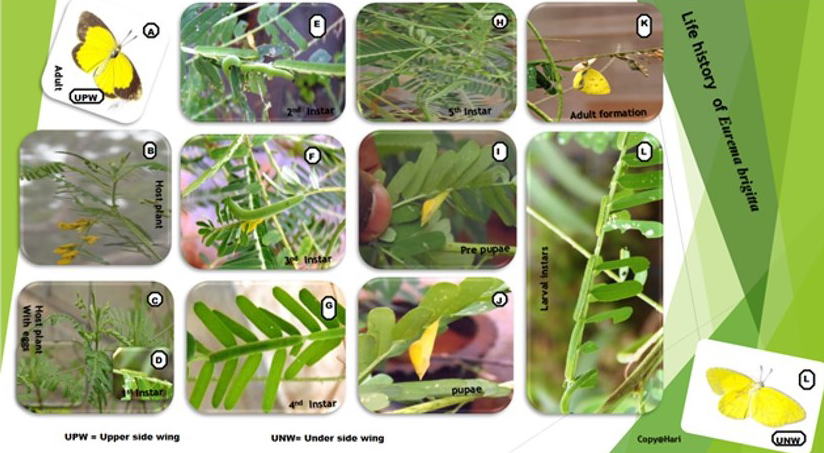
Life history and seasonality of life stages: The eggs are oval, whitish with a smooth surface, laid singly and 0.7-1.2 (1.0 ±: 0.02) mm high hatching in 3-4 days (Figure 6 and 7). The larvae pass through five instars, each of the first four instars continues to grow for 2-3 days before they cast their skin. The fifth instar lasts 3-4 days. Larval growth was continuous and increases from a length of 1.2 – 2.0 (1.8 ± 0.1) mm in the first instar to 22.0 – 24.5 (23.1± 0.11) mm in the fifth instar. The cylindrical body was pale ceramic initially but becomes dark green with black markings during its development.

Figure 7: Matting pair of Eurema brigitta
These characters persist up to the last instar; additional characters at this larval stage include the pale yellow colour and small black band on the lateral sides of the body.
Table 2: Month wise distribution of different life stages of Eurema brigitta on Cassia mimosoides in the field
|
Life cycles stage |
Jan |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
|
Eggs |
15 |
15 |
13 |
11 |
11 |
14 |
19 |
21 |
36 |
33 |
29 |
16 |
|
Larvae |
8 |
8 |
6 |
9 |
6 |
5 |
4 |
8 |
20 |
15 |
10 |
12 |
|
Pupae |
2 |
2 |
1 |
1 |
2 |
1 |
1 |
2 |
9 |
8 |
7 |
4 |
|
Adults |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
2 |
7 |
7 |
6 |
4 |
Table 3: Food consumption and utilization efficiencies of Eurema brigitta Larvae on Cassia mimosoides Leaves (Five samples)
|
Instar number |
Weight of food ingested (mg) |
Weight of faeces (mg) |
Weight gain by larvae (mg) |
GR |
CI |
AD |
ECI |
ECD |
|
I |
7.0 ± 0.08 |
0.04 ± 0.02 |
0.07 ± 0.01 |
0.79 |
17.00 |
79.7 |
2.0 |
3.68 |
|
II |
55.0 ± 0.02 |
1.20 ± 0.08 |
3.27 ± 0.06 |
0.84 |
14.60 |
82.5 |
6.9 |
7.12 |
|
III |
222.0 ± 0.24 |
12.90 ± 0.09 |
32.50 ± 0.01 |
0.78 |
6.40 |
93.4 |
16.6 |
18.60 |
|
IV |
240 ± 0.26 |
29.10 ± 0.10 |
34.60 ± 0.11 |
0.33 |
2.64 |
97.5 |
18.7 |
22.70 |
|
V |
310.0 ± 0.32 |
60.20 ± 0.17 |
59.40 ± 0.18 |
0.19 |
1.93 |
98.0 |
19.5 |
23.70 |
Growth rate (GR); Consumption index (CI); Approximate digestibility (AD); Efficiency of conversion of digested food (ECD); Efficiency of conversion of ingested food (ECI)
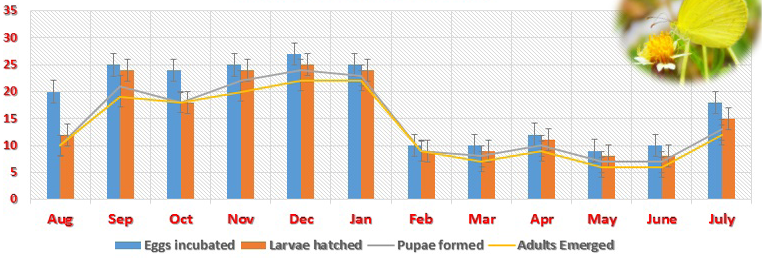
The fully grown fifth instar pupates within one day. The pupa was yellow to pale green colour, 16.0-17.5 (17.0 ± 0.1) mm long with its anterior part drawn into a snout of 4.5 – 5.4 (5.0 ± 0.02) mm long. The pupal stage lasts 6-7 days. The development success rate of different life stages in different months was high, it being 80 – 100% for Eggs, 83 -100% for Larvae and 86- 100% Pupae (Table 2). Month wise distribution of Eggs, Larvae and Pupae monitored through searches of this life stages on different plants of Cassia mimosoides indicates that the three life stages occur throughout the year in considerable frequency but with a higher density during September – November period (Table 2) and this period corresponds well with the period of their higher development success rate.
Food Consumption, Growth Rate and Utilization
The data for the weight of food consumed and weight gained by the larvae are given in Table 3. The same data could not be collected for instar I due to its small size with consequent danger in handling. The amount of food consumed increased from instar to instar, the proportion of total food consumed in instars from I to V being 0.07, 3.27, 32.50, 34.60 and 59.40% respectively. Thus, there was greatest consumption in instar V. The weight gain corresponded to the food consumption trend of the respective instars. The weight gain in instar V was 81.65% of the total larval weight. The weight of successive instars plotted against the food consumed indicated a clear relationship between these two parameters (y = 79.1x and 70.05; r = 0.9346) (Figure 9). The values of growth rate increased from I to II instar and decreased from instar II to V instar and the values varying between 0.79 to 0.84 and 0.84 to 0.19. Consumption Index (C.I.) progressively decreased from instar to instar, the values ranging between 17 and 1.93 mg/day/mg. Table 3 also includes the indices of food utilization efficiencies A.D., E.C.I., and E.C.D. The range of A.D. values was 79.7 to 98%, that of E.C.I. 2 to 19.5%
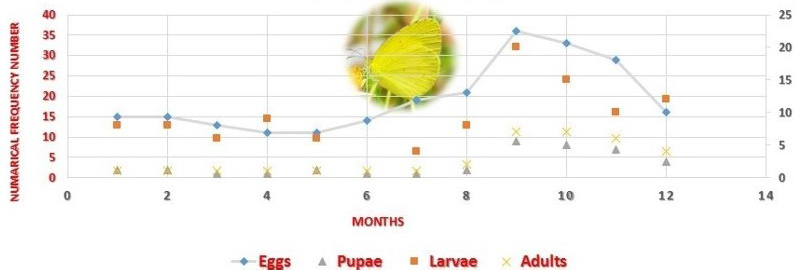
Figure 9: Month wise distribution of different life stages of Eurema brigitta on Cassia mimosoides in the field
and E.C.D. 3.68 to 23.70%. While E.C.I. and E.C.D. decreased, A.D. increased as the larvae progressed.
Quantitative data on consumption and utilization were obtained feeding the larvae with Cassia mimosoides leaves. The weight of food ingested, the weight of faceases, weight gain in larvae and food consumption growth and food utilization indices were calculated for each of the five instars (Table 3). The food consumed by instar II was 8 times more than that of instar I that by instar III was 4 times more than that of instar II; that by instar V, was a little more than that of instar IV. Thus, there was a tremendous increase in food consumption between instar II and III. The same was reflected in the weight of the larvae of instar III. The same was reflected in the weight of the larvae of instar III. The rate of intake of food relative to the mean weight of the larvae as indicated by the consumption index (CI) was high in instar I. the rate decreased slightly with instar II and then decreased steeply from instar II to instar III and rather slowly from instar III to instar IV and also from instar IV to instar V. The AD values decreased across the instars. However, the ECD and ECI values showed an increase through the successive instars. The increase was rapid from instar II to instar III which indicates that the efficiency with which digested food was converted to body substance was more at instar III.
DISCUSSION
Eurema brigitta utilizes several legumes as larval hosts and was thus polyphagic in its larval nurturing approach. It did not attain pest status of any of the host plants examined. But a species of Eurema (E. blanda) which lays eggs in large batches was reported to assume pest status on Albzzia (Wesley Jenkinson, 2013). All other species of the genus including E. hecabe and E. brigitta lay eggs singly. The strategy of single egg laying was advantageous in that the breeding females can use many host plants and different species of the host genus within a habitat and this advantage was being enjoyed by E. brigitta. Normally the larvae from such species laying eggs singly are less likely to defoliate their hosts.
The eggs hatched 3-4 days after incubation in the laboratory at around 27˚C. The individual larvae had four moults and five instars: over a period of 11-16 days. The pupal period was 6-7 days. Thus, the total period required for the egg to develop into an adult was esteemed to be 20-27 days. As temperature influences, the length of each stage of life history, the length of each life history stage and thus the total length of the life history of E. brigitta may vary in other regions of its distribution in the country depending on the prevailing temperatures. Thus, in Bengal, the larval period was longer (20 days), and the pupal period ranged between 5-25 days. Another period butterfly E. hecabae had the best development of eggs, larvae, and pupae at 27˚C (Table 4, Ramana, 2003a, 2003b). The length of different life stages of E. brigitta was compatible with that of E.hecabae, and the temperature regime favorable for reproduction of these two Pierids appears to be similar.
Kunte (2000) writes that E. brigitta may have four broods in places of climate extremes, and 12 broods in places of favorable climate. In general, the adult life span of a butterfly may extend from 7-12 days (Opler and Krizek, 1984). If this general period of life expectancy was applied in the case of E. brigitta which required 20-27 days for the completion of life history, 10-11 broods may be reasonable yearly in the environs of Kadapa. The yearlong distribution of eggs, larvae and pupae (Table 4) on the host plants in field condition supports this estimation of 10-11 broods and also shows that July–November period was most conductive for E. brigitta reproduction, and this period falls within the north-east monsoon.This peak circulation agrees with Winter-Blyth who carried the spreading of butterflies at a vicinity mostly depending on rainfall environments. The profile of food consumption and weight gain of successive instars ran on similar lines, both showing a progressive increase
Table 4: Development success of different life stages of Eurema brigitta in the laboratory
|
Life cycles stage |
Aug |
Sep |
Oct |
Nov |
Dec |
Jan |
Feb |
Mar |
Apr |
May |
June |
July |
|
Eggs incubated |
20 |
25 |
24 |
25 |
27 |
25 |
10 |
10 |
12 |
9 |
10 |
18 |
|
Larvae hatched |
12 |
24 |
18 |
24 |
25 |
24 |
9 |
9 |
11 |
8 |
8 |
15 |
|
Pupae formed |
10 |
21 |
18 |
22 |
24 |
23 |
9 |
8 |
10 |
7 |
7 |
13 |
|
Adults Emerged |
10 |
19 |
18 |
20 |
22 |
22 |
9 |
7 |
9 |
6 |
6 |
12 |
as the larvae aged. The regression of weight gain on food consumption yielded an equation: Y=79.1x with the value of 70.05 exceeding the table value of r=0.9346, (Figure 9) this indicating a linear relationship between the two variables.
Food consumption index (CI), Growth rate (GR), and approximate digestibility (AD) all behead in a similar way with the values decreasing as the larvae aged. The average values of CI and GR are 0.59 and 7.71 respectively; the first two instars recorded values higher than these averages. The percentages of AD ranged between 79.7 –98.0, the first three instars registered higher AD values than the mean. High AD values are often expected when the foliage forms the food rich in nitrogen (and water). Compared to the AD values, those of ECD and ECI are low, the ECD values ranging from 3.68-23.70 and of ECI from 2.0 - 19.5. Both ECD and ECI showed a similar pattern of increase with the age of larvae. Comparatively higher values of the last instars (ECD =22.70, 23.70; ECI =18.7, 19.5) indicates the quality of Cassia mimosoides as food for E. brigitta. Being a legume, Cassia mimosoides foliage must be rich in nitrogen and being an annual ford, its leaf water content was in the range of 75-80%.
Both larval and adult resources are the basic pre-requisites of habitat suitability for butterflies (Slansky and Scriber, 1985). E. brigitta utilizes many legumes and different floral species as adult nectar sources. Floral nectar (Table 1) was a vital source of sugars and amino acids and provides energy for flight, which was a vital force to find mates and food plants on which they lay eggs (Harris and Harris, 1997). The choice of oviposition of host species by the polyphagous E. brigitta was likely to depend on the adult requirement of floral resources as demonstrated in the choice of oviposition host species in Euphydryas chalcedona. Nectar intake might increase adult longevity, egg production and egg maturation (Waldbauer, 1968). The range of sugar concentration of floral species for E. brigitta (19-59%) corresponds well with 15- 50 % of psychophilic floral nectars. While foraging at flowers, E.a brigitta received pollen mostly on proboscis and head, thus complains about an important requirement of psychophily and provides its effectiveness in pollinating its floral species.
Adult feed on the damaged and ripened fruit helps them in obtaining proteins and carbon sources with such nutrient uptake improving egg productivity (Fischer et al., 2004). The larval food also appears to be highly nutritional as indicated by the observed values of assimilation efficiency (A.D), the efficiency of conversion of ingested food (E.C.I), and the efficiency of conversion of digested food (E.C.D) into the body substance. The chemistry of the leaf, particularly its nitrogen and water content, influences the assimilation efficiency (Pandian and Marian, 1986). The Cassia mimosoides leaves contain 2.35% nitrogen and 75.25% water (Senthamizhselvan and Murugan, 1988). Hence the observed high A.D value, mean 90.97%. Such high values are characteristic of the foliage feeders (Slansky and Scriber, 1985) and indicative of their high growth efficiency (Singhal, 1980). The values of E.C.D. and E.C.I., particularly those of the last two instars, are also relatively high, thus respectively indicating tissue growth efficiency and ecological growth efficiency, which enabled E. briggata to thrive successfully in the urban environment. The mean temperature begins to rise from later half of February and it was maximum on some days (38 – 43ºC) during May/June. Monsoon rains cool the tropical heat from June/July onwards, with the mean temperature remaining relatively high through October and thereafter decreasing to a minimum (26–32ºC) in January/February.
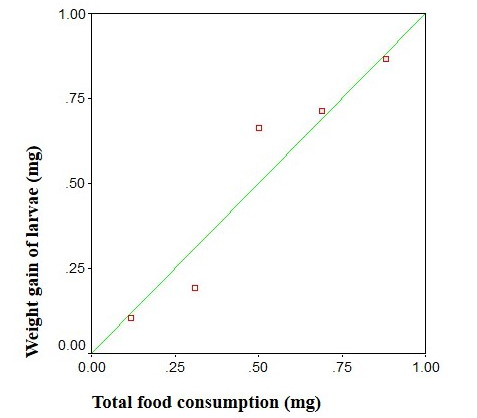
Figure 10: Normal P-P plot of FING
As it was expected of a semi environment, the maximum-minimum range in temperature was narrow, and the difference rarely exceeds 22ºC. Mean monthly temperature, relative humidity, rainfall (Figure 10) and sunlight data for the study years borrowed from the ISRO (Supercomputer facility) at the Yogi Vemana University campus, Kadapa.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest related to the work.
ACKNOWLEDGEMENTS
The Corresponding author Dr. S.P. Venkata Ramana, Department of Zoology, Yogi Vemana University greatly acknowledge the University Grants Commission (UGC), New Delhi for financial support through a Major Research Project.
AUTHOR’S CONTRIBUTION
Harinath Palem, Suryanarayana Kanike and Venkata Reddy collected the Butterfly life cycle stages and conducted captive breeding experiments in the laboratory. Venkata Ramana Sri Purushottam guided, prepared and formatted the manuscript.
REFERENCES