The Journal of Advances in Parasitology
Research Ar ticle
The Journal of Advances in Parasitology 1 (3): 35 – 38Detection of Trypanosoma Evansi by Different Methods in Bovines in Andhra Pradesh
Sirigireddy Sivajothi1*, V Chengalva Rayulu1, Bhavanam Sudhakara Reddy2
- Department of Veterinary Parasitology, College of Veterinary Science, Sri Venkateswara Veterinary University, Proddatur – 516360, Andhra Pradesh, India
- T.V.C.C. (Veterinary Medicine), College of Veterinary Science, Sri Venkateswara Veterinary University, Proddatur – 516360, Andhra Pradesh. India
*Corresponding author: sivajothi579@gmail.com
ARTICLE CITATION:
Sivajothi S, Rayulu VC, Reddy BS (2014). Detection of Trypanosoma evansi by different methods in bovines in Andhra Pradesh. J. Adv. Parasitol. 1 (3): 35 – 38.
Received: 2014–05–02, Revised: 2014–06–23, Accepted: 2014–06–25
The electronic version of this article is the complete one and can be found online at
(http://dx.doi.org/10.14737/journal.jap/2014/1.3.35.38)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
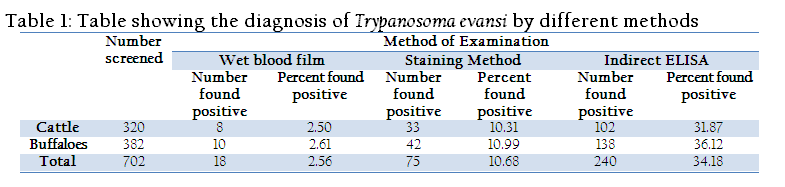
Trypanosoma evansi infection is the most important disease of bovines in India. The present work was carried out to evaluate the different diagnostic methods for field diagnosis of T. evansi infection in bovines in Rayalaseema region of Andhra Pradesh. Animals showing the clinical signs of intermittent fever, occular discharges, chronic emaciation and corneal opacity were selected in this study. A total of 702 samples from four different districts of Rayalaseema regions were collected from 2009 to 2011 for Trypanosoma evansi infection. The tests used were wet blood film (WBF), Giemsa stained smear method and Indirect Enzyme–linked immunosorbent assay (Indirect ELISA). The prevalence of T. evansi infection in cattle was detected in 8, 33, 102 out of 320 cattle by wet blood film examination, Giemsa stained smears examination and by Indirect ELISA, respectively. In buffaloes infection was recorded in 10, 42 and 138 out of 382 buffaloes by wet blood film examination, Giemsa stained smears examination and by Indirect ELISA, respectively. Among the 702 bovines 2.56 %, 10.68 % and 34.18 % were found positive by wet blood film examination, Giemsa stained smears examination and by Indirect ELISA, respectively. In this study Indirect ELISA had the higher prevalence rate as compared with other tests. Observed clinical findings in the affected bovines were correlated with Indirect ELISA results in this study.
INTRODUCTION
Trypanosoma evansi is a widely distributed flagellate protozoon that causes a disease called surra in domestic animals and is transmitted mechanically by biting flies such as Tabanus and Stomoxys spp. The incidence and the severity of the disease vary with the strain of the parasite as well as the species of host affected (Sivajothi et al., 2013a). Clinical signs in different animals vary based on the severity of the infection. Clinical signs and pathological lesions caused by T. evansi in animals are unreliable for definitive diagnosis. Diagnosis of Trypanosoma evansi infection (surra) in livestock is of considerable economic importance. Detection of trypanosomes in blood has been the “gold standard”; however, finding the organisms or establishing patency of parasitaemia has not always been possible even in symptomatic infections. As a consequence of low dose infection, the pre–patent period in Trypanosoma evansi may be longer and, even when symptoms have developed, trypanosomes may still not be demonstrable in blood, thus delaying treatment and thereby increasing morbidity and mortality (OIE, 2008). There are several techniques for parasite detection, which include direct microscopy, concentration techniques and animal inoculation (Sivajothi et al., 2012; 2013b). The most useful of these tests, in view of their sensitivity and specificity, is the enzyme immunoassay (Indirect ELISA) which is used for the diagnosis of Trypanosoma evansi infections (Sivajothi et al., 2014a). Hence the present investigation was designed to detect Trypanosoma evansi in bovines by different methods (Wet blood film method, Giemsa stained smears and Indirect ELISA) in Andhra Pradesh.
MATERIALS AND METHODS
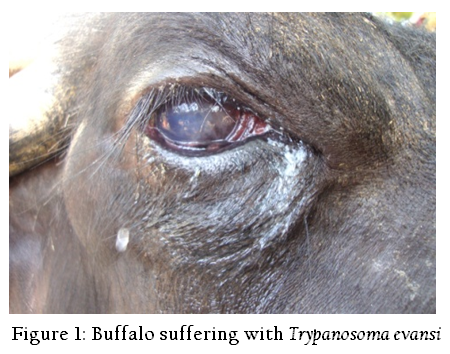
animals from the Rayalaseema region of Andhra Pradesh were screened for the infection by different methods such as wet blood film examination, Giemsa’s staining and Indirect ELISA (Enzyme–linked immunosorbent assay). Animals showing the clinical signs of intermittent fever, occular discharges, chronic emaciation and corneal opacity were selected in this study (Figure 1). Blood was obtained from the peripheral ear vein and jugular vein from suspected animals. Trypanosomes were recognized by their movement among the red blood cells (RBCs) in wet blood film examination. Blood samples collected into separate sterile vials without Ethylenediaminetetraacetic acid (EDTA) for serum collection. Serum samples were collected in sterile vials. Few drops of 1:10,000 sodium azide solution was added to the serum samples and stored at –20 °C until use.
Wet blood film examination was done by placing a droplet of blood on a clean microscope slide and covering with a cover slip (22 × 22 mm). The blood is examined microscopically at (400×) total magnification approximately 50–100 fields per slide. A drop of blood was taken in the center on one end of a clean glass slide for smear preparation as suggested by Benjamin (Benjamin 1986). The blood smears were prepared and dried by waving in the air. The blood smears were fixed in absolute methyl alcohol for 3 min and stained with Giemsa’s stain method of staining as described by Benjamin (Benjamin 1986). A drop of cedar wood oil was placed on the stained smear and the slide was examined under the oil immersion lens (1000×) of the microscope for the presence of Trypanosoma evansi. Approximately 50–100 fields of the stained thin smear are examined, before the specimen was considered to be negative. Even after a trypanosome has been detected, approximately 20 extra fields were investigated to determine if more than one species was present. The sharp extremities of the smears were extensively explored, because of their capillary properties; trypanosomes may be concentrated at this place (OIE 2004; Sivajothi et al., 2013b).
Isolate of T. evansi collected from the field case was maintained in the Wistar rats for bulk harvest of parasites. At the high of parasitaemia in rats, the rats were bled by heart puncture and the blood was collected and used for separation and purification of Trypanosoma evansi by using DEAE (Diethyl amino–ethyl cellulose anionexchange column chromatography).Whole cell lysate (WCL) antigen was prepared from purified trypanosomes. Protein concentration of the WCL Ag of T. evansi prepared in the present study was estimated as per Lowry et al. (1951) and it was adjusted to 1.0 mg /ml in PBS, pH 8.0 and stored at –200C in 1.0 ml aliquots. The hyper immune sera (HIS) was raised in two healthy New Zealand white rabbits, raised against WCL Ag was confirmed by agar gel precipitation test and counter immune electrophoresis. Pre immunized serum of these experimental rabbits was also stored at –20ºc till use as negative control serum for standardization of Indirect ELISA (Sivajothi et al., 2014a).
RESULTS AND DISCUSSION
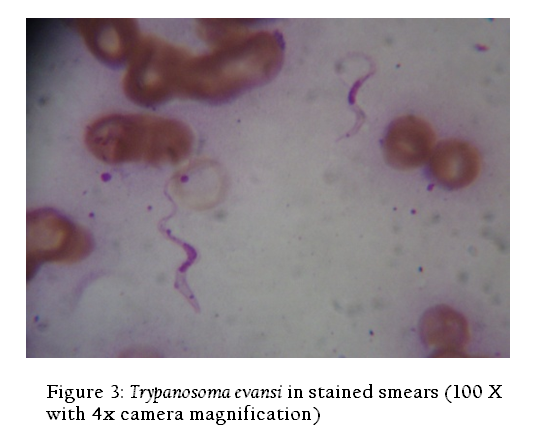
In wet blood film examination Trypanosomes were recognized by their movement among the red blood cells (RBCs) (Figure 2). Examination of the Giemsa stained smear had Trypanosoma evansi; it was monomorphic in character, slender in shape, having an undulating membrane with a well developed free flagellum present outside the cell as reported previously by Soulsby (1982) (Figure 3).Serum samples were examined for the presence of T. evansi antibodies by Indirect ELISA (Figure 4). T. evansi was detected by using the different methods. The results of different diagnostic tests adopted for the detection of T. evansi in bovines in Andhra Pradesh was presented in Table 1. In the wet blood film examination 8 (2.50 %) cattle were positive out of 320 animals screened whereas 10 (2.61 %) buffaloes were positive out of 382 screened. In the blood smear staining methods, 33 (10.31%) cattle were positive out of 320 animals screened whereas 42 (10.99%) buffaloes were positive out of 382 screened. The ELISA detected antibodies against T. evansi in 102 (31.87%) cattle out of 320 screened whereas 138 (36.12 %) buffaloes were positive out of 382 screened.
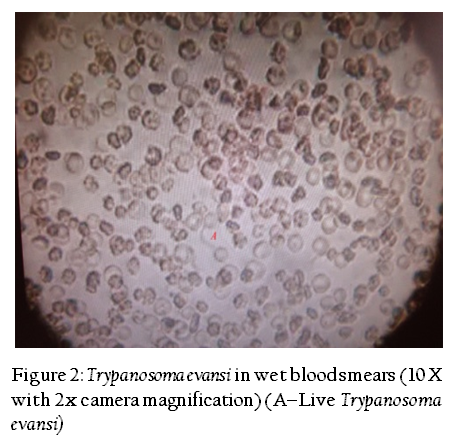
Figure 2: Trypanosoma evansi in wet blood smears (10 X with 2x camera magnification) (A–Live Trypanosoma evansi)
Prevalence of T. evansi can be correlated with the vector population and favorable conditions for vector breeding, species of the host, host immune status etc. The WBF method is simple, inexpensive and gives immediate results. Depending on the trypanosome size and movements a presumptive diagnosis can be made of the trypanosome species. The diagnostic sensitivity of the method is generally low but depends on the examiner’s experience and the level of parasitaemia. Giemsa stained blood smear examination is simple and relatively inexpensive, but results are delayed because of the staining process. Trypanosomes are easily recognized by their general morphology, but may be damaged during the staining process. This may make it difficult to identify the species.
One obvious reason for low number of positive samples by WBF was the inherent low sensitivity of the test. Similar observations have been made by numerous workers over past decades in India (Desquesnes et al., 2009). Sinha et al. (2006) reported T. evansi prevalence in 52.69 and 52.31 per cent of cattle and buffaloes in Patna. Rayulu et al. (2009) recorded an overall prevalence of T. evansi in 2.47, and 34.98 per cent of domestic animals in Haryana by WBF and Ag– ELISA, respectively. Molina et al. (2000) found 1.3% and 9.0% positive by WBF and Ab– ELISA, respectively. The number of positive animals by ELISA in the region should be higher than those detected by WBF. Because, latent infections with low parasitemia are common in cattle, buffaloes and camels, it is likely that many samples were found negative by WBF and staining technique. Antigenaemia due to release of the antigens from the parasites hiding in some tissues has also been speculated (Sivajothi et al., 2014b). Another reason why WBF figures were low could be the indiscriminate treatment of suspected animals for trypanosomosis based only on signs and symptoms. The recently treated animals would be declared positive by immunological tests. Parasite antibodies may remain in blood circulation for about few weeks to months after treatment (Wernery et al., 2001). Therefore, this fact would necessitate getting reliable history of the animal that received anti–trypanosome treatment during past few weeks before sample collection to make indirect–ELISA more dependable.
According to this study, parasitological techniques like wet blood film examination, stained blood smears examination were frequently used for diagnosis of T. evansi infections had low sensitivity. The serological tests identified the animals with sub–patent infections which could not be detected by parasitological methods. During this study, all samples those were found positive by parasitological methods were positive as well by Indirect ELISA when used for the same samples. Few sample were negative by parasitological methods but were found positive when using Indirect ELISA. This Indirect ELISA will make it possible to detect infections in bovines in the very early stages where microscope examination is unclear and to monitor groups of animals after trypanocidal treatment. Furthermore, this will not only be beneficial for diagnosis but also useful for epidemiological study and designing rational Trypanosomosis control program. Present study concludes that diagnosis of clinical and subclinical T. evansi infection in bovines by Indirect ELISA is more reliable than the remaining two tests.
ACKNOWLEDGEMENTS
The authors acknowledge the authorities of Sri Venkateswara Veterinary University, Tirupati for providing facilities to carry out this research. Authors are also thankful to the field veterinarians who assisted in collection of blood samples.
CONFLICT OF INTEREST
No conflict of interest.
REFERENCES
Benjamin MM (1986). Outline of Veterinary Clinical Pathology, 3rd ed., the Iowa State Univ. Press, Ames, Iowa, USA. 1986
Desquesnes M, Bossard G, Thevenon S, Patrel O, Lepetitcolin E, Hollzmuller P, Berthier D, Jacquiet P, Cuny G (2009). Development and application of an antibody–ELISA to follow up a Trypanosoma evansi outbreak in a dromedary camel herd in France. Vety. Parasitol. 162: 214–220.
http://dx.doi.org/10.1016/j.vetpar.2009.03.033
PMid:19372008
Lowry OH, Rosebrough NJ, Farr AL, Randall R (1951). Protein estimation with the folin–phenol reagent. J. Biol. Chem. 193: 265–275.
PMid:14907713
Molina JM, Ruiz A, Juste MC, Corbera JA, Amador R, Gutierrez C (2000). Seroprevalence of Trypanosoma evansi in dromedaries (Camelus dromedaarius) from the Canary Islands (Spain) using an antibody Ab–ELISA. Prev Vet Med. 47: 53–59.
http://dx.doi.org/10.1016/S0167-5877(00)00157-4
OIE (2004) Surra (Trypanosoma evansi) in: "Manual of standards for diagnostic tests and vaccines". Office International des Epizooties World Health Organization for Animal Health, Paris, 5th edition pp: 891–900.
OIE (2008). Trypanosoma evansi infections (including surra) In: OIE Terrestrial Manual 2008. Office International des Epizooties World Health Organization for Animal Health, Paris. Pp: 352– 360.
Rayulu VC, Singh A, Chaudhri, SS (2009). Evaluation of parasitological and monoclonal antibody based assays in detection of Trypanosoma evansi in animals. Indian J Anim Sci. 79(10): 978–981.
Sinha BS, Verma SP, Mallik KP, Samantary S, Kumar B, Kumar RP (2006). Study on epidemiological aspects of bovine Trypanosomosis in some districts of Bihar. Vet Parasitol. 20(1): 69–71.
Sivajothi S, Rayulu VC, Reddy BS (2012). Development of Slide Enzyme Linked Immunosorbent Assay (SELISA) for Detection of Trypanosoma evansi Infection in Bovines. J.A.V.R.. 2: 15–17.
Sivajothi S, Rayulu VC, Malakondaiah P, Sreenivasulu D (2013a). Colloidal Dye Immunobinding Assay for Detection of Trypanosoma evansi Antibodies in Animals. Int. J. Livest. Res. 3(3): 48–56.
Sivajothi S, Rayulu VC, Reddy BS (2013b). Haematological and biochemical changes in experimental Trypanosoma evansi infection in rabbits. J. Parasit Dis. DOI 10.1007/s12639–013–0321–6.
http://dx.doi.org/10.1007/s12639-013-0321-6
Sivajothi S, Rayulu VC, Malakondaiah P, Sreenivasulu D (2014a). Diagnosis of Trypanosoma evansi in bovines by indirect ELISA. J Parasit. Dis. DOI 10.1007/s12639–014–0465–z.
http://dx.doi.org/10.1007/s12639-014-0465-z
Sivajothi S, Rayulu VC, Sujatha K, Reddy BS (2014b). Study of Histopathological Changes in Experimental Trypanosoma evansi Infected Rats. Proc. Zool. Soc. 2014a DOI 10.1007/s12595–014–0104–9.
http://dx.doi.org/10.1007/s12595-014-0104-9
Soulsby EJL (1982). Helminthes, Arthropods, and Protozoa of Domesticated Animals. 7th; ed. Bailliere and Tindal, London. 1982; 532–33.
Wernery U, Zachariah R, Mumford JA, Luckins T (2001). Preliminary evaluation of diagnostic tests using horses experimentally infected with Trypanosoma evansi. Vet J. 161: 287–300.
http://dx.doi.org/10.1053/tvjl.2000.0560
PMid:11352486