Advances in Animal and Veterinary Sciences
Research Article
Assessment of Genotoxic Potential in Rats Collected from Bathinda District of South-west Punjab
Shasta Kalra*, Gurinder Kaur Sangha
Department of Zoology, Punjab Agricultural University (PAU), Ludhiana 141004, India.
Abstract | The wide spread use of the chemicals in agriculture has caused environmental pollution and potential health hazards. The present study was carried out to evaluate the genotoxicity in rats inhabiting Bathinda. Rats were brought to laboratory and separated according to species. The animals were dissected and the liver was processed for microsomal degranulation. The frequency of micronucleated erythrocytes in femoral bone marrow preparations was also evaluated. Increase in microsomal degranulation was observed among Bathinda rats of all the species as compared to control rats. Maximum microsomal degranulation was in Tatera indica (5.6%) followed by Rattus rattus (5.3%)and Bandicota bengalensis (2%) collected from Bathinda. Microsomal degranulation in rats inhabiting Ludhiana district was 1%, 2.4% and 3% respectively. Frequency of micronucleated cells was significantly increased in all field rats inhabiting Bathinda district as compared to Ludhiana district. The present study substantiates the genotoxic potential of environmental contaminants in rats inhabiting south- west region of Punjab.
Keywords | Environmental contaminants, Carcinogenesis, Genotoxicity, Micronucleus, Microsomal degranulation.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | December 28, 2017; Accepted | January 31, 2018; Published | March 15, 2018
*Correspondence | Shasta Kalra, Department of Zoology, Punjab Agricultural University (PAU), Ludhiana 141004, India; Email: shastakalra@yahoo.com
Citation | Kalra S, Sangha GK (2018). Assessment of genotoxic potential in rats collected from bathinda district of south-west Punjab. Adv. Anim. Vet. Sci. 6(3): 108-112.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.3.108.112
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Kalra and Sangha. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
There is a great deal of concern regarding the hazard potential of human exposure to toxic substances and carcinogens as well as infectious agents in the environment. Exposure to various toxic chemicals has become an increasingly recognized source of illness in human and animals worldwide (Mostafalou and Abdollahi, 2012). The wide spread use of the chemicals in agriculture has caused environmental pollution and potential health hazards (Agarwal and Sharma, 2010). Numerous environmental and industrial chemicals are known to induce cytogenetic damage in human peripheral blood lymphocytes (Angerer et al., 2007).
Pesticides have become integral part of agriculture in Punjab. Bathinda district in Punjab, an important belt of the country, irrigated by canal water, grows largely cotton and rice crop, the two crops known for excessive use of pesticides (Puri et al.,1999). Agrochemical processes in the waterlogged agricultural area with calcareous soil and use of phosphate fertilizers are also favoured sources for deterioration of ground water quality in this district (Bhalla et al., 2011). Failing assess to clean and safe drinking water and excessive usage of pesticides has resulted in deteriorating health of people of this region (Thakur et al., 2008; Chadha and Prabha, 2013; Mittal et al., 2014). Environmental contamination due to excessive use of pesticides has become a great concern to the public and to environmental regulatory authority (Ghosh and Philip, 2006).
Environmental contaminants including pesticides possess a potential genotoxicity in occupationally exposed populations where they induced some types of cancers (Bolognesi, 2003; Rusiecki et al., 2004). Many environmental pollutants are chemical carcinogens and mutagens with the capacity of causing DNA damage (Mostafalou and Abdollahi, 2012). Degranulation of microsomes in liver is considered a potential indicator for carcinogenicity (Purchase et al., 1978). Micronucleus and other anomalies are biomarkers of genotoxic events and chromosomal instabil
Table 1: Microsomal degranulation
| Rattus rattus | Bandicota bengalensis | Tatera Indica | ||||
| Control | Bathinda | Control | Bathinda | Control | Bathinda | |
| RNA (µg/g liver) | 813±0.02 | 506.66±0.01 | 506.6±0.03 | 280±0.01 | 906±0.01 | 560±0.01 |
| Protein (µg/g liver) | 12480±0.23 | 13600±0.01 | 8640±0.01 | 12960±0.42 | 10240±0.03 | 16480±0.13 |
| RNA: Protein ratio | 0.065±0.03 | 0.037±0.02* | 0.06±0.09 | 0.07±0.01 | 0.08±0.009 | 0.034±0.001* |
| Control-RNA: Protein ratio | 0.024 | 0.053* | 0.03 | 0.02 | 0.01 | 0.056* |
| % microsomal degranulation | 2.4% | 5.3% | 3% | 2% | 1% |
5.6% |
Values are Mean ± SE,
*Significant difference at (p≤0.05) as compared to control
Table 2: Frequency of micronucleated cells
| Total number of examined cells | No. of micronucleated cells | Frequency of micronucleated cells | |
|
Rattus rattus- Control |
200 | 5.00±1.15 | 2.50 |
| Bathinda | 200 | 13.33±1.20* | 6.66* |
|
Bandicota bengalensis- Control |
200 | 4.33±1.45 | 2.16 |
| Bathinda | 200 | 14.00±1.00* | 7.00* |
|
Tatera indica- Control |
200 | 2.66±0.66 | 1.33 |
| Bathinda | 200 | 13.00±0.88* |
6.50* |
Values are Mean ± SE,
*Significant difference at (p≤0.05) as compared to control
ity (Singh et al., 2013). Higher micronucleated cell counts were observed in workers exposed to car paints and lead (Singh et al., 2013; Kassie et al., 2000).
Genetic monitoring of populations exposed to potential carcinogens is an early warning for genetic disease or cancer. It allows identification of risk factors at early stages when control measures could still be implemented (Kassie et al., 2000). Thus the present investigation was carried out to assess the genetic damage in rats inhabiting Bathinda region of Punjab.
Materials and Methods
During the study, the field rats i.e., Rattus rattus, Bandicota bengalensis and Tatera indica were trapped from fields of Bathinda district of South West region of Punjab. Same species of rats were also collected from PAU and adjoining areas and they served as control rats. Animals were brought to laboratory, separated according to species and observed for morphological symptoms. Approval of Institutional Animal Ethical Committee, Guru Angad Dev Veterinary and Animal Science University (GADVASU), Ludhiana was obtained for the usage of animals vides letter no. 3901-35 dated 06-08-2012.
Microsomal Degranulation
After the sacrifice, the animals were dissected and was excised, cleaned of adhering tissue and 0.5 g of liver was finely chopped and homogenized in 0.225 M sucrose tris (ST) buffer (pH 7.4) in chilled conditions and processed for microsomal degranulation. Tissue homogenates were centrifuged for 20 min at 9000 rpm at 4°C, the post mitochondrial supernatant collected and mixed with 0.5 g calcium chloride. After that the tubes were kept in ice for 20 min, centrifuged at 4°C, 10,000 rpm for 20 min. The pelleted microsomes were resuspended in 0.225 M ST buffer (pH 7.4). Proteins and RNA were estimated from the supernatant as per standard methods (Lowry et al., 1951; Munro and Fleck, 1966).
Micronucleus Assay
The frequency of micronucleated erythrocytes in femoral bone marrow preparations was evaluated according to the recommended procedure (Schmid 1975; Alder et al., 1991). After the sacrifice, the femurs were desiccated out, cleaned from muscular tissue and both cartilaginous epiphyses were cut off. The marrow was flushed out with 2 ml phosphate buffer saline (PBS) into a centrifuge tube, using a clean syringe. The samples were centrifuged at 2000 rpm for 5 minutes. Following centrifugation, the supernatant was discarded and the cells resuspended in a drop of PBS. The suspensions were spread on slides and air dried. At least five slides were prepared from each animal, fixed in methanol, stained in Wright stain followed by Giemsa stain, and rinsed in distilled water. The slides were then observed under light microscope. About 200 cells from each slide were studied to know the frequency of micronucleated cells.
Statistical Analysis
All statistical comparisons were presented as the mean ± standard error of mean (S.E.M). Comparisons were made between control and Bathinda rats belonging to different species on computer using t-test. A “P” value of 0.05 was selected as a criterion for statistically significant differences.
Results
Microsomal Degranulation Test
The observations recorded indicate that exposure to environmental contaminants can induce microsomal degranulation. Comparatively higher percentage of microsomal degranulation was recorded in all the species of rats of Bathinda district when compared to Ludhiana rats (Table 1).The percentage of microsomal degranulation ranged from 1% -3% in Ludhiana rats. In rats collected from Bathinda region the percentage of micromal degranulation ranged from 2-5.6%. Microsomal degranulation was found to be maximum in Tatera indica (5.6%) followed by Rattus rattus (5.3%) and Bandicota bengalensis (2%) rats collected from Bathinda region.
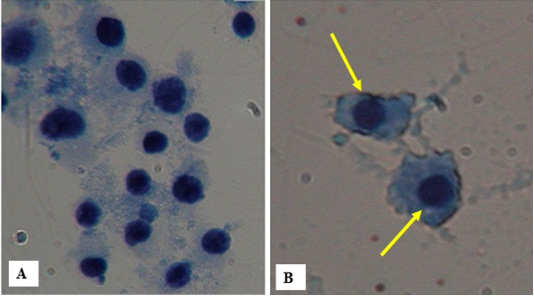
Figure 1: A) Bone marrow cells of control rats Rattus rattus, Bandicota bengalensis and Tatera indica with no micronucleus B) Bone marrow cells of different species of rats clearly showing the increase in number of micronucleated cells.
Micronucleated Assay
The frequency of micronucleated cell was found to be more in all the rats collected from Bathinda as compared to their control (Table 2, Figure 1). Number of micronucleated cells in Rattus rattus collected from Bathinda district showed a significant increase (13.33±1.20) as compared to rats of PAU (5.00±1.15). In Bandicota bengalensis control rats, the number of micronucleated cells were 4.33±1.45 as compared to 14.00±1.00 which was observed in rats collected from Bathinda district. Number of micronucleated cells in Tatera indica collected from Bathinda district also showed a significant increase (13.00±0.88) as compared to control rats (2.66±0.66).
Discussion
The carcinogenic potential of environmental contaminants was assessed by measuring the detachment of ribosomes from rough endoplasmic reticulum (RER). Researchers have demonstrated that electriphiles of a carcinogen can disrupt ribosome membrane interaction in rough microsomes by their attack on nucleophilic components of the reticular membrane ribosome complex, involving in protein synthesis for export from cytosol. Lack of exported proteins can adversely affect signal transduction across plasma membrane possibly leading to events at molecular levels and thus, incidence of carcinogenesis (Mehta and Hundal, 2014).
Our results are in consonance with (Sahambi and Dhanju, 2005) the study where monocrotophos showed maximum microsomal degranulation (25%) in rat liver, followed by dimethoate (18.07%) and methyl parathion (13.44%). It was reported (Purchase et al., 1978) that if the degranulation of microsomes in liver is more than 5%, then the chemical has a potential for carcinogenicity as the ribosomes get released from rough endoplasmic reticulum. As microsomes carry the polysomes, which are involved in biosynthesis of proteins and also have number of enzymes to metabolize the foreign components like pesticides, their degranulation is indicative of their lost integrity (Sahambi and Dhanju, 2005). Rate of metabolism of pesticide is determined by factors, which establish the rate of entry of pesticides into endoplasmic reticulum of the liver, the major site of detoxification (Hutson, 1981). In vivo microsomal degranulation in liver, kidney, testis, lungs, small intestine mucosa, bone marrow cells and spleen cells was reported after oral administration of 1/12, LD50 dose of malathion and 1/10th LD50 of phenthoate (Bakshi, 1996).
The micronucleus test as applied to polychromatic erythrocytes in bone marrow of rodents is also an efficient alternative to metaphase chromosome analysis for the detection of cytogenetic damage in- vivo in mammalian somatic cells (Hammam and Abdel-Mottaleb, 2007). It is sensitive, non-invasive, cost- efficient and reliable test to locate chromosomal mutations (Singh et al., 2013). MN frequencies in the exposed workers were found to be three times higher than in non-exposed workers (Singh et al., 2013). Significant increase in the induction of polychromatic erythrocytes micronucleus was also observed in rat bone marrow cells after pesticide exposures (Hammam and Abdel-Mottaleb, 2007). Significantly higher micronucleated cell counts were reported in workers exposed to car paints and lead than control (Roth et al., 2002). Profenofos at all doses also showed an increase in the frequency of micronucleated polychromatic erythrocytes (D’ Souza et al., 2005) (Hammam and El-Khatib, 2004). Dose- dependent increase in % degranulation in hepatic fraction was observed in female albino rats fed with arsenic at permissible dose levels (Mehta and Hundal, 2014).
Conclusion
The increase in genetic damage in rats indicates the potential genetic hazards posed by excessive use of pesticides in Bathinda district. The long-time over-use of pesticides appears to be a major cause for prevalence of various diseases in cotton cultivated districts of the Malwa region of Punjab. Providing safe drinking water and uncontaminated foods are the foremost requirements. There is an urgent need to reduce morbidity and mortality related to pesticide poisoning through review and improved pesticide policies. Implementation of sustainable epidemiological surveillance and monitoring of pesticides poisoning in clinical settings and communities is necessary. Strengthening of community programs about the safe use of pesticides can minimize the risks of intentional and unintentional pesticide poisoning.
Acknowledgments
The authors are very thankful to Head, Department of Zoology, Punjab Agricultural University Ludhiana, Punjab for providing the necessary facilities to carry out the research work.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors contribution
Both the authors made substantial contributions to design, acquisition of data, analysis and interpretation of data; Authors participated in drafting the article or revising it critically for important intellectual content; and gave final approval of the version to be submitted and any revised version.
References






