Advances in Animal and Veterinary Sciences
Research Article
Clinico-biochemical Examination of Pregnant Toxemic Goats with Special Emphasis on the Enzymatic and Hormonal Pattern
Noura Elshahat Abdel-Rhman Attia1, Rasha Thabet Metwaly Alam2*
1Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Sharkia, Egypt; 2Department of Clinical Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Sharkia, Egypt.
Abstract | Pregnancy toxemia (PT) is a metabolic stress syndrome, occurring in does during the late stage of gestation; especially those with twins or triplets. This study was aimed to evaluate the biochemical and hormonal alterations associated with clinically pregnant toxemic goats. Forty clinicallyhealthy and toxemicpregnant goats were used. Pregnant goats with early toxemia had anorexia, depression, dullness and scanty feces, with pronounced acetone odor from the mouth, recumbency, and nervous manifestations were noticed in the untreated and progressed cases. Striking metabolic alterations in goats with PT showed a significant decrease in the serum glucose level while beta-hydroxybutyrate (βHB) showed a significant increase. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), and alkaline phosphatase (ALP) showed a significant increase. Also, goats with PT showed a significant increase in the blood urea nitrogen and creatinine levels. Moreover, the hormonal profile showed a significant increase in the serum cortisol and insulin, while triiodothyronine (T3), thyroxine (T4) and growth hormone were decreased significantly in pregnant toxemic goats. Histopathologically, the liver showed fat globules distending the nuclei of the hepatocyte to the periphery with moderate lipidosis. These results suggest that the main cause of death among goats with PT may be due to the hepato-renal dysfunction. The increase in energy metabolites and hormonal alterations constitutes an important tool for the diagnosis and determination of the magnitude of clinically pregnant toxemic goats.
Keywords | Pregnant goats, Toxemia, Biochemical, Enzymes, Hormones
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 21, 2016; Accepted | January 26, 2017; Published | February 17, 2017
*Correspondence | Rasha TM Alam,Department of Clinical Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Sharkia, Egypt; Email: rashaalam@ymail.com
Citation | Attia AEN, Alam TMR (2017). Clinico-biochemical Examination of Pregnant Toxemic Goats with Special Emphasis on the Enzymatic and Hormonal Pattern. Adv. Anim. Vet. Sci. 5(2): 62-69.
DOI | http://dx.doi.org/10.14737/journal.aavs/2017/5.2.62.69
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Attia and Alam et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Peri-parturient losses of goats are of a great economic impact on goat enterprises. Mammal pregnancy is a critical period in a female life as she considered responsible for obtaining a new progeny, that must be survive, have the strength to develop and reproduce in the future. The serious effect of this period increases when the dam is pregnant with 2 or more feti as nutritional requirements rise during the last third of gestation. Pregnancy toxemia is a drastic problem in pregnant small ruminants, caused by an abnormal metabolism of carbohydrates and fats, occurred at the final stage of pregnancy “last 4-6 weeks of gestation” (Brozos et al., 2011). Additionally, it is the most common metabolic disease of small ruminants, and considered one of the main destructive diseases which threaten the productivity in sheep and goats (Rook, 2000). The disease is usually seen in goats car¬rying multiple feti, and it is caused by a negative energy balance in the late gestation (Van Saun, 2000; Kulcsar et al., 2006). Furthermore, most of the fetal growth occurs during this period, so increase the demand of feto-placental unit from glucose as a main source of energy (Lindsay, 1973). During the last gestation period, approximately 33 to 36% of the blood glucose is directed into the placental unit to satisfy its energy demands (Hay et al., 1983). Sheep and goats pregnant with twins or triplets required 180 to 240% more energy than that carrying a single lamb (Ermilio and Smith, 2011). Moreover, hypoglycemia and hyperketonaemia are the most common obvious biochemical disturbances in pregnancy toxemia (Harmeyer and Schlumbohm, 2006; Radostits et al., 2008).
The present study was carried out in an attempt to obtain further investigations on the clinical signs and biochemical analysis with special reference to the endocrine response (insulin, cortisol, T3, T4 and growth hormone) in clinically healthy and toxemic pregnant deos, as well as the ultrasonographic, postmortem and histopathological examination of the liver which help in the diagnosis and prognosis of pregnancy toxemic goats.
MATERIAL AND METHODS
Animals
Twenty healthy pregnant does of nearly similar age (3-4 years old), and gestation period (1-3 weeks pre-lambing) was admitted to the Veterinary Hospital of the Faculty of Veterinary Medicine Zagazig University, Sharkia Province, Egypt, were examined and used as a control group. In addition, another twenty pregnant doesshow the clinical signs of pregnancy toxemia (1-3 weeks pre-lambing)as clinical diseasedcases were examined at the animalhospital, Faculty of Veterinary Medicine, ZagazigUniversity, Egypt. All goats were polyparus and pregnant with 2-3 fetus as observed by ultrasound. The study was approved by the ethical committee of Zagazig University in accordance with the guidelines of the National Institutes of Health (NIH) for the Care and Use of Animals.
Clinical Examination
All does under investigation were subjected for clinical examination according to the methods of (Sherman and Robinson, 1983) including the rectal temperature, heart and respiratory rates, and rumen contractions were measured. Inspection of the visible mucous membranes and lymphnodes of the animal was also performed using routine clinical diagnostic methods. Body condition score was assessed using a 5-point scale (1–5) as described by (Villaquiran et al., 2012). The animals were evaluated visually, via palpation of the lumbar vertebrae and sternumregion.
Ultrasonography Examination
B-mode ultrasound was used for examination of the liver, it can be applied on the right side of the animal body, starting at the 12th intercostal space and moving cranially to the 7th intercostal space using a 5 MHz linear transducer (Braun, 2004).
Blood, Urine And Tissue Sampling
Blood samples were collected from the jugular vein in both healthy and diseased pregnant goats. Blood was allowed to flow into a clean glass tube, left about 30 minutesto clot at room temperature, then centrifuged at 3000 rpm for 15 min, and the serum was separated. One part of the serum was stored at 4°C (< 48hrs) until used for assaying βHB and glucose concentrations, and another part was stored at -20°C for analysis the other biochemical parameters.Fresh urine samples were collected through the spontaneous urination of the animals and from the bladder of recently dead cases (about 5ml was collected in a clean and dry plastic container), for biochemical examination. Liver samples were collected from the recently dead pregnant toxemic goats and fixed at 10% neutral buffered formalin for histopathological examination.
Biochemical Assays
Serum samples were used for measuring of ALT and AST according to (Reitman and Frankel, 1975), and the serum ALP according to (Kind and King, 1954). Gama glutamyltransferase (GGT) was assessed as described by (Szasz ,1969). Serum levels of insulin, cortisol, T3, T4, and growth hormone were measured by radioimmunoassay (Reuter et al., 1978). Serum glucose level was measured according to (Sugiura and Hirano, 1977), and β-Hydroxybutyrate (βHB) level was determined by a kinetic enzymatic method using nefa randox kits. Serum creatinine was determined according to (Heinegard and Tiderstrom, 1973), while urea level was estimated according to (Tabacco et al., 1979). All the commercial kits used were provided by Biomerieux, and Spin react,Spain.
Urine Analysis
Urinalysis reagent strips were used to detect the ketone bodies and measure the pH of the urine samples according to the directions of the manufacturer (Roche combur urine strips®, Boehringer Monnheim, Germany). Hold the reagent strip in a horizontal position, dip it into each urine sample individually, remove it after one minute, and compare it to the chart colors to record the results.
Histopathological Examination
Liver samples from pregnant toxemic goats were collected and fixed at 10% buffered neutral formalin. Liver sections of (5-μm) were deparaffinized, prepared, and stained with hematoxylin and eosin for histopathology (Suvarna et al., 2013).
Statistical Analysis
The results were subjected to Student’s t-test according to the method described by (Snedecor and Cochran, 1989) by using the Statistical Analysis System (SAS) software. The results were considered statistically significant when *p < 0.05,**p < 0.01, ***p< 0.001.
RESULTS
Physical Examination And Clinical Signs
The healthy examined pregnant goats not showed any abnormal clinical signs, while the physical examination and clinical findings of goats with pregnancy toxemia are shown in (Table 1). In the early stage, does exhibit altered appetite, depression, dullness, reluctance to move (Figure 1), ruminal stasis, grinding of teeth and scanty feces. The animals tend to have poor muscle control and frequent urination also noticed. In advanced cases the animals become recumbent, anuria, pronounced acetone odor from the mouth and in the urine and showing neurological signs in form of blindness, head pressing, tremors, convulsions and champing of the jaw with deviated head.In the terminal stage, the animals lay down in lateral recumbent position, and become dehydrated, comatose and eventually die (Figure 2).
Table 1: The incidence and percentage of pregnancy toxemia in goats under investigation
|
Risk factor |
Number of diseased goats |
Percent |
|
No of feti: have 2 feti 3 feti |
12 8 |
60% 40% |
|
Body condition score: Too fat >4 Too thin <2.5 Normal |
6 12 2 |
30% 60% 10% |
|
Age of animal: All affected goats were 3-4 years |
20 |
100% |
|
Concurrent diseases: With concurrent diseases Without concurrent diseases |
15 5 |
75% 25% |
|
Season: Extreme winter Extreme summer Other season |
9 5 6 |
45% 25% 30% |
|
Stress factor " poor quality of ingested feed, transportation, lack of exercise" |
All diseased goats are subjected to one of these stress factors |
100% |
Body temperature, heart rate and respiration rate were within the normal level in the early stage, however, in the advanced stage, heart and respiration rates decreased,alsoa snuffling respiration observed in some cases.Respiration becomes labored and body temperature subnormal in the final stage of the diseases. Ruminal movements were decreased in some cases and complete cessation recorded in other cases (Table 2).

Figure 1: (a) Goat with pregnancy toxemia “good body condition’, depression and dullness but in standing position, (b) Depressed pregnancy toxemic goats in sternal recumbence, (c) Goat with recumbence and nervous manifestation “deviation of head and neck”, (d) Comatosed goat.

Figure 2: (a) Recumbent dehydrated pregnancy toxemic goat, (b) notice sunken eye, (c) the disease developed rapidly and animal was slaughtered and found 3 alive kids but death occurred within 10 minutes.
Biochemical Findings
The alteration in the selected biochemical parameters in the present study is shown in (Figure 3 and 4). Pregnancy toxemic goats showed a significant decrease in the serum glucose level in goats with PT compared to healthy pregnant goats, while the serum βHB showed a significant increase in gestating does with PT compared with clinically healthy gestating ones. In comparison to healthy pregnant goats, there were significant increases in the serum hepatic enzymes ALT, AST, ALP and GGT in goats with PT. Serum urea and creatinine levels showed a significant increasein pregnancy toxemic goats when compared with
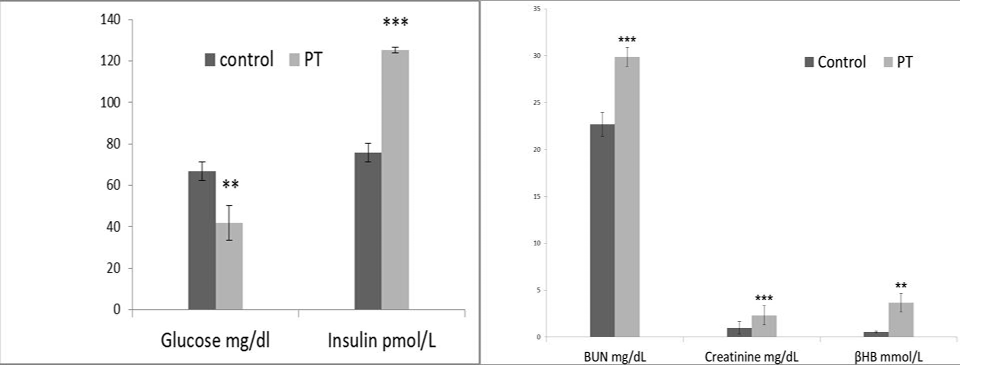
Figure 3: Effect of pregnancy toxemia on the serum levels of glucose mg/dl, insulin IU/l, urea mg/dl, creatinine mg/dl and BHB mmol/l in goats.Note: ** Significant at p <0.01and *** Significant at p < 0.001.
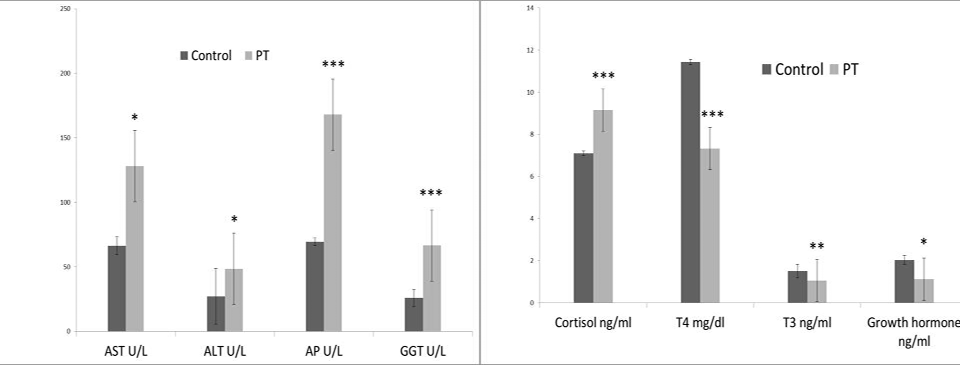
Figure 4: Effect of pregnancy toxemia on the serum levels of ALT, AST, ALP, GGT, cortisol, T3, T4 and growth hormones in goats.Note:* Significant at p < 0.05; ** Significant at p <0.01 and *** Significant at p < 0.001.
control ones. Urine analysis showed ketonuria and aciduria in goats with PT.
Toxemia during the late stage of pregnancy alters the hormonal pattern in the body. Goats with PT showed a significant increase in the serum insulin and cortisol concentration, with a significant decrease in the T3, T4, and growth hormones.
Ultrasonography Findings
Liver ofthe healthy pregnant goats showed hypo-echogenicity (Figure 5a), while pregnancy toxemic goats revealed an increase in the echogenicity (Figure 5b) mainly “fatty liver”.
Postmortem And Histopathological Findings
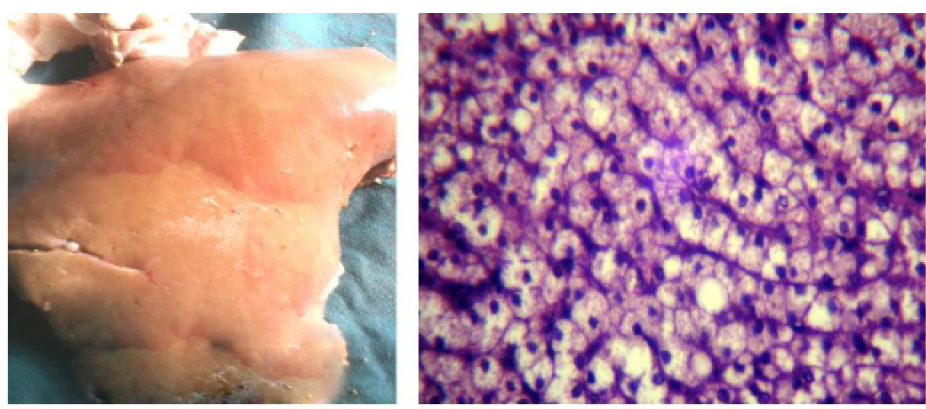
In fresh dead cases,the liver was pale yellow, slightlyswollen and friable (Figure 6a), while the histopathological examination of the liver (Figure 6b) revealed fat globules distending the hepatocyte nuclei to the periphery with moderate lipidosis.
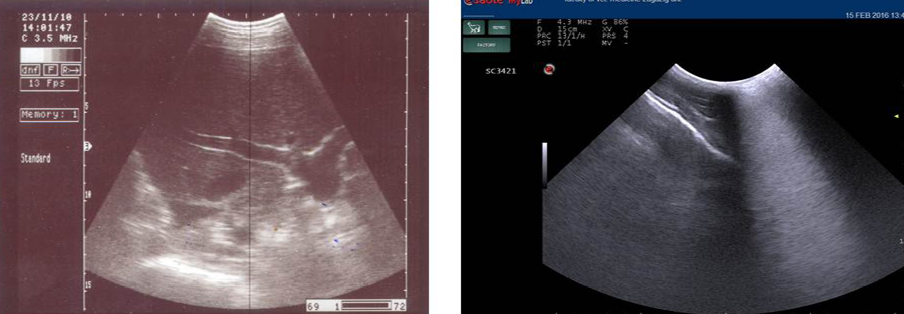
Figure 5: Ultrasonography image of normal liver appeared as homogenous hypo-echogenic structure (a) and that of pregnancy toxemic goat (b), notice the increased echogenicity of the liver “arrow” and faint appearance of hepatic vasculature
Table 2: Clinical findings and examination of the clinically healthy and pregnant toxemic goats.
|
Clinical parameters |
Clinically healthy pregnant goats |
Pregnancy toxemic goats |
|
Appetite |
Normal |
Decreased- anorexia |
|
State of animal |
Alerted |
Depressed |
|
Posture |
Normal in standing or recumbent position |
Mainly recumbent either in sternal or lateral position. |
|
Sweet acetone odour from the mouth |
Absent |
Detectable |
|
Feces |
Normal |
Scanty |
|
Nervous manifes- tation |
Absent |
Vary from grinding onteeth- Muscular tremors- Champing jaw- blindness- Deviated head and neck |
|
Ruminal movement |
2-3/2 minutes |
1/ minute or completely absent |
|
Rectal temperature |
39.7 |
In early stage: normal In terminal stage: slight decreased. |
|
Heart rate |
80/min. |
In early stage: slight decreased. In terminal stage: decrease. |
|
Respiration rate |
23/min. |
In early stage: normal. In terminal stage: decrease. |
DISCUSSION
Caprine pregnancy toxemia (PT) is a disease occurs
commonly in doe carrying more than one fetus, due to increase the fetal demand for glucose, especially during the second half of pregnancy (Radostitis et al., 2007). In the present study, we found that, the common risk factors of pregnancy toxemia are associated with the mother’s body condition either too low body condition score < 2.5 (thin does), or too high body condition score >4 (fat does) these results were similar to those previously reported by (Scott, 2007). Our study revealed that, all affected animals with caprine PT were 3-4 years, these results were in agreement with (Bostedt and Hamadeh, 1990) and (Abdelaal et al., 2013). Moreover, the stress factors such as sudden changes and poor quality feed, transportation, extreme weather, and infectious diseases have a rule in the occurrence of the disease which confirmed by a significant increase in the serum stress marker (cortisol).
Physical examination and clinical findings of goats with pregnancy toxemia, in the early stage, does exhibited altered the appetite, depression, reluctance to move, ruminal stasis, grinding of teeth and scanty feces, while in the advanced cases, the animals become recumbent, anuria, pronounced acetone odor from the mouth and urine, also showing neurological signs which may be due to impairment of glucose metabolism and inability of the nerve cell to utilize glucose due to high levels of cortisol which producing hypoglycemic encephalopathy. Also, may be due to the production of iso-propyle alcohol, which resulted from theacetoacetic acid breakdown in the rumen (Radostits et al., 2007). Moreover, the toxic effect of acetoacetic acid reduces the brain oxygen consumption, and producing the nervous signs (Sackran et al., 2004). These changes are irreversible in this cases and the animal not respond to the treatment (Rook, 2000; Witsch et al., 2012). In the terminal stage, the animals lay down in lateral recumbent position, dehydrated, comatose and eventually die. These results were in agreement with (Rook, 2000; Hefnawy et al., 2011; El- Dee, 2012). In the advanced stage of the disease, heart and respiration rates were decreased, this may be attributed to the toxemic states which is accompanied by peripheral circulatory failure and death (Van Saun, 2000).
Regarding to the biochemical alterations in goats with pregnancy toxemia, in the present study, we founded that there is a significant reduction in the serum glucose level in goats with PT, which may be attributed to decrease in the hepatic gluconeogenesis (Mills et al., 1986), with increase the energy requirements of the pregnant goats that carries two feti (Voicu et al., 1993), also decrease the endogenous glucose production may be developed as a result of decrease calcium levels, which occur during the period of pregnancy (Schlumbohm and Harmeyer, 2003), and may be due to the hyperketonemia produces adverse effect onenergy balance and glucose metabolism (Harmeyer and Schlumbohm, 2006). These results were in agreement with (Hefnawy et al., 2010; Gonza´ lez et al., 2011; Abdelaal et al., 2013). Moreover, βHB is the main detectable ketone body in the blood resulted from the hepatic reduction of the most of acetoacetate by hydroxybutyrate dehydrogenase enzyme producing the higher blood βHB concentration (Grohn et al., 1983). Our results revealed a significant increase in the serum βHB in the gestating does with PT, which could be attributed to the disturbance in the carbohydrate and fat metabolism leading to increase the lipolysis of tissues fat and release the long chain fatty acids which were converted by the hepatocytes to ketone bodies “hepatic ketogenesis” (Rook, 2000; Hefnawy et al., 2010) and this resulted as a compensatory mechanism in response to carbohydrate deficiency (Reece, 2004) in pregnant does with more than one fetus. Moreover, reduced the ability of the late pregnant doe to utilize ketone bodies so impair the disposal of ketone bodies (Harmeyer and Schlumbohm, 2006). Similar results obtained by (Hefnawy et al., 2010; Gonza´ lez et al., 2011).
Regarding the enzymatic alterations in caprine PT, our results showed a significant increase in the serum hepatic enzymes ALT and AST, which could be attributed to the damage in hepatic cells, increasing its membrane permeability, leading to release the cellular enzymes to circulation (Kaneko et al., 1997), this damage resulted from increase the metabolism of the body fats due to energy deficiency which in turn increases the circulating free fatty acids that reach the liver and subsequently induce fatty infiltration.Also,our results showed a significant increase in ALP in pregnancy toxemic goats, it may be attributed to inflammation of the hepatic epithelial cells surrounding the bile ducts or may be fromrenal origin. These results agree with (Sargison et al., 1994). According to our results, the serum glucose and ALP levels were useful indices for the diagnosis and prognosis of caprine PT. (Abd El-Raof and Ghanem, 2006) proved substantially higher blood АSТ, АLT and АР in goats with PT.
Serum GGT appears to be a specific and sensitive indicator of hepaticinjury, making it a good diagnostic aid even over serum AST (Blackshaw, 1978), so increased the activity of this enzyme indicate acute and chronic liver disease (Cebra et al., 1997). Pregnancy toxemic goats in the present study showed a significant increase in the serum GGT, which was attributed to damage in liver parenchyma. Our results are confirmed by ultrasonographic examination of the liver in goats with PT which showed an increase in the echogenicity mainly “fatty liver” (Tontis and Zwahlen, 1987) which confirmed by postmortem findings in dead cases,the liver was pale yellow, slightly swollen and friable, similar results recorded by (Albay et al., 2014; Abba et al., 2015). Histopathologically, the liver showed fat globules distending the hepatocyte nuclei to the periphery with moderate lipidosis. Similar results obtained by (Abba et al., 2015). In the present study, the serum urea and creatinine levels showed a significant increase in pregnancy toxemic goats, which could be attributed to damage in the glomerular epithelial cells due to sever acidosis and ketonemia associated with PT (Nassif et al., 2005), leading to decrease the glomerular filtration rates so decreasing the removal of nitrogenous waste product (urea and creatinine) from the blood. Also, fatty infiltration in the renal tubular epithelium of the kidney in pregnancy toxemic goats may responsible for renal dysfunction (Tontis and Zwahlen, 1987). These results were coincided with those previously reported by (Ismail et al., 2008; Hefnawy et al., 2011).
Urine analysis showed ketonuria and aciduria, which occurs due to increase the glucose demand than the dietary supply in pregnancy leading to increase the mobilization of long chain fatty acids from adipose tissues and a marked rise in circulating non esterified fatty acid and ke-tone bodies, which in return, descend in urine (Harmeyer and Schlumbohm, 2006). Our results are in agreement with (Gonza´ lez et al., 2011; Albay et al., 2014).
According to the previous studies, there is a little information available regarding to the endocrine pattern in pregnancy toxemic goats, our results revealed a significant increase in the serum insulin concentration in goats with PT, which could be attributed to the highly increase in the free fatty acids stimulate the release of insulin which may have an inhibitory role of ketogenesis (Hefnawy et al., 2010), moreover, increase the level of serum insulin may be a cause help in the developing of hypoglycemia. Also, we found a significant increase in the serum cortisol level which may be attributed to increase the adrenal output under stress condition that subjected to it the pregnant goats and increase the glucose demand (Ford et al., 1990). Furthermore, there is a significant decrease in the serum T4 level in goats with PT, which may be attributed to the excessive secretion of cortisol as there is a negative correlation between free T4 and cortisol (Sartorelli et al., 2000), similar results recorded in ewes (Kulcsar et al., 2006). Also, our results revealed a significant decrease in the growth hormone level as its secretion is inhibited by high cortisol and free fatty acid concentrations (Jarrett et al., 1974). In the present study, death of the dam may be attributed to reduction inthe liver and kidney function which confirmed by the biochemical analysis.
Conclusion, from the findings of the present study, pregnancy toxemia had an economic impact on goat industry, especially due to severe irreversible organ damage. Blood BHB and glucose concentrations are the primary parameters for monitoring the negative energy balance and ketosis. The hormonal and enzymatic profile analysis should be implemented as important tools for diagnosis and prognosis of caprine pregnancy toxemia, also the liver and kidneys are involved in the pathogenesis of it.
ACKNOWLEDGMENTS
We thank Dr. Haytham Ali, a lecturer of Pathology, Faculty of Veterinary Medicine, Zagazig University for his technical assistance in tissue processing for histopathological examination.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
Author’s contribution
All authors contributed equally to this work.
References