Advances in Animal and Veterinary Sciences
Research Article
Ameliorative Effect of Garden Cress (Lepidium sativum L.) Seeds Ethanolic Extract on High Fat Diet-prompted Non-alcoholic Fatty Liver Disease in the Rat Model: Impact on 3-Hydroxy-3-methylglutaryl-Coenzyme A Reductase and Vascular Endothelial Growth Factor
Ibrahim A. Ibrahim1, Abeir A. Shalaby1, Hoda ML. Abdallah2, Nermin F. El Zohairy2, Hoda I. Bahr1*
1Department of Biochemistry, Faculty of Vet. Medicine, Suez Canal University Ismailia 41522, Egypt; 2Department of Biochemistry, Animal Health Research Institute, Zagazig, Egypt.
Abstract | Non-alcoholic fatty liver disease (NAFLD) occurs by excrescent hepatic fat accumulation without the excessive intake of dietary alcohol and can progress to non-alcoholic steatohepatitis (NASH) and cirrhosis. This study explored the ameliorative effect of ethanolic extract of garden cress seeds (EEGS) in rat NAFLD model. Rats were divided into four groups. Group 1 (Control, Standard diet + distilled water) fed on the standard diet. Group 2 (HFD + distilled water) and 3 (HFD + EEGS fed highfat diet (HFD) for 12 weeks. Beginning from the seventh week, groups 3 (HFD + EEGS) and 4 (Standard diet + EEGS) fed EEGS 400mg/kg b.w/day till the end of the experiment. HFD-fed rats had increased body weight, serum liver enzymes leakage, hyperlipidemia, and an occurrence of hepatic oxidative stress, lipid peroxidation, NAFLD, and NASH. Compared to the HFD group,EEGS treatment lowered TAG, LDL-C, TC, downregulation of hepatic HMGCR, and VEGF expression and prevented from obesity, NAFLD, NASH, and fibrosis. EEGS treatment in the HFD group increased GSH, SOD, CAT activities, and a decreased MDA and nitric oxide level. The data concludes that, daily administration of EEGS 400mg/kg b.w for six weeks is safe and possess hepatoprotective, antioxidant, hypolipidemic, anti-obesity, anti-angiogenic, and anti- steatosis characteristics in HFD-induced NAFLD.
Keywords | High-fat diet. NAFLD. NASH, Lepidium sativum L. HMGCR. VEGF
Received | June 10, 2020; Accepted | June 25, 2020; Published | July 12, 2020
*Correspondence | Hoda Ibrahim Bahr, Department of Biochemistry, Faculty of Vet. Medicine, Suez Canal University Ismailia 41522, Egypt; Email: [email protected]
Citation | Ibrahim IA, Shalaby AA, Abdallah HML, El-Zohairy NF, Bahr HI (2020). Ameliorative effect of garden cress (Lepidium sativum L.) seeds ethanolic extract on high fat diet-prompted non-alcoholic fatty liver disease in the rat model: Impact on 3-hydroxy-3-methylglutaryl-coenzyme a reductase and vascular endothelial growth factor. Adv. Anim. Vet. Sci. 8(s1): 1-10.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s1.1.10
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Ibrahim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The liver is responsible for lipid metabolism (Henao-Mejia et al., 2012). The non-alcoholic fatty liver disease (NAFLD) develops by an accumulation of excrescent hepatic fat without the intake of excessive alcohol. Non-alcoholic steatohepatitis (NASH) is a severe form that can progress to cirrhosis and hepatocellular carcinoma (Rafiq et al., 2009; Machado and Diehl., 2016; Zhou et al., 2018). NAFLD is correlated with metabolic diseases such as obesity, insulin resistance, atherosclerosis, and cardiovascular disease (Wójcik-Cichy et al., 2018). Feeding on the high-fat diet (HFD) results in hepatic redox imbalance mediating lipid peroxidation that promotes the progression of NAFLD (Rolo et al., 2012; Kakimoto and Kowaltowski, 2016).
Recently, herbal medicine has been found to prevent and treat many diseases (Karam et al., 2017). Garden cress (Lepidium Sativum L.) belongs to the Brassicaceae family and is native to Egypt and West Asia. It possesses proteins, vitamins, carbohydrates, omega-3 fatty acids, iron, phytochemicals, and flavonoids (Nehdi et al., 2012; Emhofer et al., 2019). Garden cress (GC) seeds extract possesses antioxidant, hypoglycemic, hepatoprotective, cardioprotective, antidiarrheal, and anticancer activity (Al-Sheddi et al., 2016; Raish et al., 2016; Ullah et al., 2019). Feeding GC seed oil supplementation for 60 days than natural oil in rats, Umesha and Naidu (2015) reported higher levels of hepatic tocopherols, catalase, and glutathione peroxidase activity. Following oral supplementation of 20%GC methanol seed extract (w/v) for 28 days in streptozotocin-induced diabetic rats, Qusti et al. (2016) observed an increased renal and pancreatic activity of SOD, CAT, GSH, and a decreased MDA contents. A pre-exposure of GC seeds extract (25 µg/ml) showed cytoprotective effects against H2O2-induced toxicity in HepG2 (Al-Sheddi et al., 2016). Another study revealed that pretreatment of GC seeds extract (150 and 300 mg/kg) down-regulates TNF-α, IL-6, and IL-10, MPO, caspase3 activity, and up-regulates BCl2 expression in D-galactosamine/ lipopolysaccharideinduced hepatotoxicity in rats (Raish et al., 2016). Though much studied, there is a paucity of research on the free radical scavenging and antioxidant properties of ethanolic extract of garden cress seeds (EEGS). Therefore, while assessing hepatic oxidative stress status, and expression of HMGCR and VEGF mRNA, this study explored the hepato-ameliorative effect of EEGS in rat NAFLD model.The phytochemical screening of EEGS was also carried out to determine the contents of total phenolic, flavonoids, vitamin C, and elucidate DPPH radical scavenging activity.
Materials and Methods
Rats model
Forty adult male albino rats (120-130gm, six weeks old) were obtained from The Laboratory Animal House of National Research Center, Dokki, Giza, Egypt. The rats were housed in the research building of the Faculty of Veterinary Medicine, Suez Canal University. Rats were fed standard pellet animal diet and water ad libitum. The whole experiment involving animal-related activities were done in accordance with the “Guide for the Care and Use of Laboratory Animals». NAFLD model was established by HFD that consisted of standard diet (355 gm/kg), lard (310 gm/kg), casein (250 gm/kg), cholesterol (10 gm/kg), corn oil (10 gm/kg), DL-Methionine (3 gm/kg), vitamins and mineral mix (60 gm/kg), sodium chloride mix (1 gm/kg), and yeast powder (1 gm/kg) (Srinivasan et al., 2005).
Plants
Garden cress seeds were obtained from Horticulture Department, Agricultural Research Center, Dokki, Giza, Egypt. Plants were identified by the Faculty of Pharmacy, Department of Pharmacognosy, Cairo University, Egypt.
Preparation of ethanolic extract of garden cress seeds (EEGS)
The GC seeds (500 g) were washed by distilled water, dried using hot air oven (40–60 oC), and ground by an electric grinder. The ground seeds were soaked in 96% ethanol for 24 hr, and filtered. Ethanol was added for another 12 hours and using sterile filter paper, filtered the extract. The filtrate was dried in an oven at 50ºC and stored at 4°C until used (Samson et al., 2012).
Experimental regimens
Rats were divided randomly into four groups, (n=10 in each group). Control group (group 1) rats were fed standard pelleted diet and water. After six weeks of the experiment, rats were provided 1ml distilled water using gastric gavage daily till 12 weeks.HFD group (group 2) rats offered a high-fat diet (HFD) for 12 weeks. After six weeks of the experiment, rats were provided 1ml distilled water using gastric gavage daily till 12 weeks. HFD+EEGS group (group 3) rats were fed HFD for 12 weeks. After six weeks from the beginning of the experiment, rats were offered EEGS (400mg/kg/day) using gastric gavage until the end of the experiment (Kamani et al., 2017). EEGS (group 4) rats were fed the standard pelleted diet for 12 weeks. After six weeks from the beginning of the experiment, rats received EEGS (400mg /kg/day) using gastric gavage until the end of the experiment.
Handling of blood and tissue samples
After 12 weeks, rats were fasted overnight, and euthanized. Blood samples were centrifuged for serum separation. Livers samples were dissected and divided into three parts. The first portion homogenized in phosphate-buffered saline (PH=7.4), centrifuged at 3000xg for 15 minutes, and the supernatant was collected for oxidative stress markers analysis. The second part was fixed in 10% phosphate-buffered formalin for histopathological examination as per the method described previously (Bancroft and Gamble, 2008). The third portion was kept at -80°C for HMGCR and VEGF gene expression analysis.
Body weight gain
The weight of each rat was recorded weekly, and weight gain was assessed following previous guidelines (Feldman-Winter et al., 2018).
Measurement of serum liver enzyme activity and lipid profile
Commercially available kits were used to determine Alanine Aminotransferase (ALT) activity, Cat. No. TR18503, Thermo Fisher Scientific, USA, Aspartate Aminotransferase (AST) Cat. No. TR70121, Thermo Fisher Scientific, USA), total cholesterol (TC), Cat. No.1105, Vitro Science, Egypt), triacylglycerol (TAG) Cat. No. 702040050, Vitro Science, Egypt, and high density lipoprotein- cholesterol (HDL-C) Cat. No. 282000005, Vitro Science, Egypt following manufacturer guidelines. The concentration of low density lipoprotein- cholesterol (LDL-C) (Davidson and Rosenson, 2009) and very low density lipoprotein- cholesterol (VLDL- C) (Zhao et al., 2017) was followed the previously described protocols.
Assay of oxidative stress markers
Serum nitric oxide (NO) concentration was determined using Griess’s reagent (Sigma-Aldrich, Steinheim, Germany) as per the method described previously (Jabłonska et al., 2007). Commercial ELISA kits were used to assess the concentration of reduced hepatic glutathione (GSH) (GSH ELISA kit, Cat. No. E02G0367, Blue Gene Biotech, China), superoxide dismutase (SOD) (SOD ELISA kit, Cat. No. MBS036924, MyBioSource, USA), catalase (CAT) (CAT ELISA kit, Cat. No. E0242r, ELAab, China) and MDA (MDA ELISA kit, Cat. No. E0156Ra, Bioassay technology laboratory, China) following the guidelines of the manufacturer.
Real-time expression of HMGCR and VEGF mRNAs
Liver total RNAs were extracted (RNeasy Mini Kit, Qiagen) according to the manufacture protocol. Total RNA was converted to cDNA (RevertAid Reverse Transcriptase (Thermo Fisher, Catalog number: EP0441). Quantification of expressed 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) (Morral et al., 2007) and vascular endothelial growth factor (VEGF) (Peng et al., 2014) was conducted using primers, probes (Metabion, Germany) (Table 1) and Quantitect SYBR green PCR kit. The PCR was performed on the Stratagene MX3005P instrument (Applied Biosystems). Thermal conditions were 94 °C for 15 min, followed by 40 cycles of each of 94 °C for 15 s and 60 °C for 30 s. The target was normalized to an endogenous reference (β-actin) and using 2−ΔCt in the strata gene MX3005P software, quantified (Bahr et al., 2019a).
Assessment of phytochemical properties of EEGS
The value of total phenolics in EEGS was evaluated by Folin-Ciocalteau protocol and described as gallic acid equivalents (GAE/g) extract according to a previous method (Dandge et al., 2011). The amount of total flavonoid was measured and described as quercetin equivalents (mg QE /g) (Kumar and Roy., 2018). Antioxidant activity was detected by DPPH (2,2-diphenyl-2-picryl hydroxyl free radical) assay, and the IC50 value was calculated (Norshazila et al., 2010).
HPLC analysis for identification of total phenolic, flavonoids and vitamin C content
A C18 column was used for screening and separation of compound constituents in a volume of 10ul in HPLC (Agilent 1260 HPLC series. The mobile phase contained water (A) and 0.02% tri-floro-acetic acid in acetonitrile (B) the rate of 1 ml/min. At wavelength (280 nm) and temperature (35°C), rate of flow was adjusted as 0 min (80% A), 0–5 min (80% A), 5-8 min (40% A), 8-12 min (50% A), 12-14 min (80% A), and 14-16 min (80% A). checked Phenolic, flavonoids, and vitamin C were detected compared to retention times against standards, and the contents were measured using the area below standards peak (Croci et al., 2009). For the identification of phenolic compounds, standards as gallic acid, caffeic acid, coumaric, syringic acid, vanillin, cinnamic acid and salicylic acid, ferulic, and chlorogenic acid were employed. For identification of flavonoids, standard as catechins, kaempferol, rutin, rosmarinic acid, hesperetin, quercitrin, apigenin, and quercetin were used. Vitamin C standard was applied for the detection of its concentration.
Statistical analysis
Values were statistically analyzed via SPSS, version 20. Data was set as mean± SD. The comparison was made through a One-way Analysis of Variance (ANOVA) followed by Duncan Multiple Range Test. The significance was taken at P<0.05.
Results
Effect of EEGS on weight gain in HFD- treated groups
The body weight gain of HFD-fed rats was more than the control group. Daily administration of 400mg/kg EEGS for six weeks counteracted HFD- induced obesity. No difference was observed between EEGS and the control group (Table 2).
Effect of EEGS on serum liver enzymes in HFD- treated groups
We observed a higher serum activity of ALT and AST in HFD- treated rats than the control group. On the other hand, these biologic markers decreased significantly following EEGS treatment. However, no differences were observed between EEGS and the control group (Table 2).
Effect of EEGS on lipid profile in HFD- treated groups
Compared to the control group, Consumption of high-fat diet caused hyperlipidemia, and this can clearly evidence by a significant increase in serum TC, TAG, and LDL-C, VLDL-C levels. Contrary to this, the administration of the EEGS prevented hyperlipidemia compared to with the HFD- treated rats. No difference was observed between EEGS and the control group (Table 2).
Table 1: Primers Sequences for HMGCR and VEGF.
| Reference | Primer sequence (5'-3') | Gene |
| TCCTCCTGAGCGCAAGTACTCT | Rat ß-actin | |
| GCTCAGTAACAGTCCGCCTAGAA | ||
| GGCTCTGAAACCATGAACTTTCT | VEGF | |
| GCAGTAGCTGCGCTGGTAGAC | ||
| CAGCACTGTCGTCATTCATTTCC |
HMG COA reductase |
|
| ACATTCCACCAGAGCGTCAAGG |
Table 2: The effect of EEGS on weight gain, serum liver enzymes, and lipid profile in HFD- treated groups.
| EEGS | HFD+EEGS | HFD | Control | Parameters |
|
122.78±8.16c |
172.51 ±5.79b |
220.32±6.65a |
125.94±10.06c |
Weight gain(gm) |
|
91.32±6.14c |
116.77 ±0.80b |
177.20±4.84a |
94.79±3.77c |
TC (mg/dl) |
|
73.98±5.02c |
82.81±1.22b |
171.98 ±6.47a |
74.89 ±4.00c |
TAG (mg/dl) |
|
40.62±1.89a |
31.83±1.45b |
21.16±1.32c |
41.15±1.72a |
HDL-C (mg/dl) |
|
36.01±3.36c |
68.38±1.68b |
121.71 ±3.61a |
38.66±1.36c |
LDL-C (mg/dl) |
|
14.68±1.14b |
16.55±0.24b |
34.33 ±1.35a |
14.97±.80b |
VLDL-C (mg/dl) |
|
26.80±1.82c |
32.86±4.37b |
51.83±1.37a |
26.63±1.34c |
ALT (IU /L) |
|
41.13±0.97c |
50.46±1.62b |
68.22±1.42a |
41.40±1.79c |
AST (IU /L) |
Values represent as (Mean ± S.D). Means with different superscript letters within the same raw are significantly different at P<0.05. HFD: high fat diet, EEGS: ethanol extract of garden cress seeds.
Table 3: The effect of EEGS on oxidative stress markers in HFD- treated groups.
| EEGS | HFD+EEGS | HFD | Control | Parameter |
|
42.95±0.38c |
48.25±0.32b |
56.47±0.48a |
43.15±0.40c |
NO (µmol/L) |
|
42.59±1.64a |
38.18±1.77a |
29.64±0.91b |
41.58±2.48a |
GSH content (Pg/g liver) |
|
44.59± 0.38a |
38.18±1.23b |
29.64±1.48c |
46.71±0.78a |
SOD activity (U/g liver) |
|
59.91±0.32a |
58.07±0.40a |
41.04±0.38b |
60.27±0.27a |
CAT activity (U/g liver) |
|
19.78±0.17c |
30.26±0.17b |
45.04±0.28a |
19.17±0.13c |
MDA content (mmol /g liver) |
Values represent as (Mean ± S.D). Means with different superscript letters within the same raw are significantly different at P<0.05. HFD: high fat diet, EEGS: ethanol extract of garden cress seeds.
Table 4: Determination of antioxidant capacity, total phenolic, and flavonoids concentration of EEGS.
|
DPPH radical scavenging activity (μg/ml) |
Total flavonoids (mg QE /g) |
Total phenolic (mg GAE /g) |
| 176.18± 0.63 |
4.79 ± 0.24 |
11.03 ± 0.75 |
Effect of EEGS on oxidative stress markers in HFD- treated groups
A higher serum nitric oxide level and lower hepatic GSH level, as well as activities of SOD and CAT were observed in HFD fed rats than control (Table 3). The EEGS administration exhibited antioxidant activity. No difference was observed between EEGS and the control group.
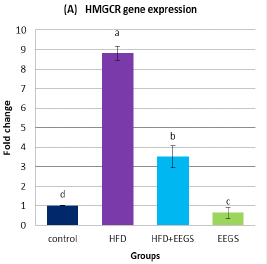
Effect of EEGS on the expression of HMGCR and VEGF in HFD- treated groups
Real-time PCR analysis demonstrated the upregulation of HMGCR (Figure 1A) and VEGF (Figure 1B) expression in HFD-fed rats compared to control. Contrary to this, following HFD feeding, EEGS administration resulted in lower expression of HMGCR and VEGF than HFD-treated rats. Interestingly, oral administration of 400mg/kg EEGS alone to rats for six weeks also revealed a lower HMGCR and VEGF expression than the control group.
Histological analysis of liver
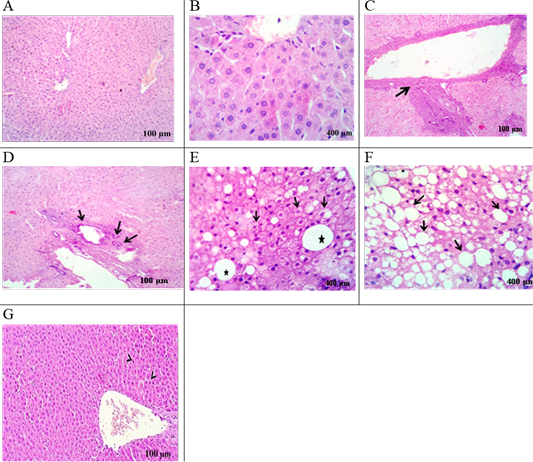
The liver of the HFD group displayed a mild proliferation of bile duct, congestion with pyknotic hepatocytes, infiltration of mononuclear inflammatory cell, fibrosis (Figure 3C), and biliary hyperplasia (Figure 3D) were noted compared to control rats. Hydropic degeneration and fatty change with fatty cyst (Figure 3E), and progressive steatosis were also recorded (Figure 3F). Following HFD feeding, rats treated with EEGS exhibited retained hepatic lobules integrity with randomly dispensed apoptotic cells (Figure 3G).
Values represent as (Mean±S.D). Means with different superscript letters within the same raw are significantly different at P<0.05. HFD: high fat diet, EEGS: ethanol extract of garden cress seeds. (A) HMGCR: 3-hydroxy-3-methylglutaryl-coenzyme A reductase. (B) VEGF: vascular endothelial growth factor. Fold-change for HMGCR and VEGF gene expression was normalized versus expression of β-actin.
Control group: liver section in (A), (B) viewing normal histomorphological structures. HFD- group: liver section in (C) showing Portal fibrosis (arrow), (D) biliary hyperplasia (arrows), (E) hydropic degeneration and fatty change (Arrows) with fatty cyst (star), (F) extensive fatty replacement of hepatic parenchyma (arrows). HFD+EEGS group: liver section in (G) evoking normal hepatic parenchyma in line with randomly dispensed apoptotic cells (arrow heads).
Determination of total phenolic, flavonoids, and DPPH radical scavenging impact of EEGS.
Table 4 demonstrate total flavonoids content as 4.79 ± 0.24 mg QE mg/ g. Total phenolic content was found to be 11.03 ± 0.75 mg GAE /g. Antioxidant activity, measured by DPPH assay indicated a lower IC50 (176.18± 0.63 μg/ml), which corresponds to the higher antioxidant capacity of EEGS.
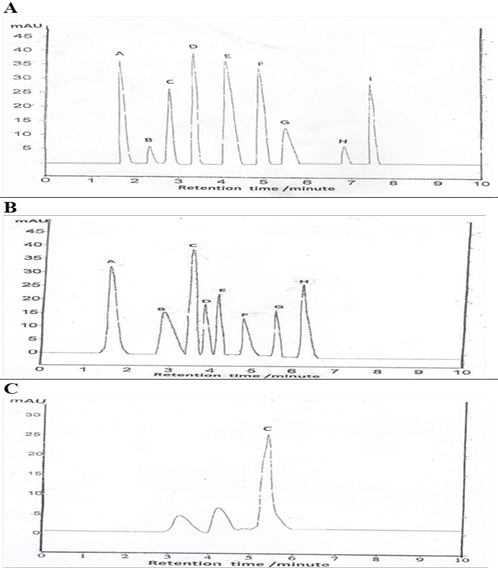
Figure 3: HPLC analysis for identification of total phenolic, flavonoids and vitamin C content of EEGS.
(A): Values represent total phenolic compound (A- gallic acid, B- vanillin, C- cinnamic acid, D- caffeic acid, E- p-coumaric acid, F- ferulic acid, G- chlorogenic acid, H- salicylic acid, I- syringic acid). (B): Values represent total flavonoids compound (A-Catechins, B- kaempferol, C-rutin, D- rosmarinic acid, E- Quercitrin, F- hesperetin, G- apigenin, H- quercetin). (C): Values represent vitamin C content.
HPLC analysis for identification of EEGS total phenolic and flavonoids and vitamin C level
HPLC analysis for EEGS phenolic compound yielded a higher value for caffeic, followed by gallic, p-coumaric, ferulic, syringic, cinnamic, chlorogenic, salicylic acid, and vanillin orderly (Table 5, Figure 3A). Identification of total flavonoids content revealed the presence of a higher value of rutin, followed by catechins, quercetin, quercitrin, rosmarinic acid, kaempferol, apigenin, and hesperetin (Table 5, Figure 3B). Vitamin C content is represented in (Table 5, Figure 3C).
Discussion
In this study, HFD for 12 weeks induced- hyperlipidemia, obesity, and increased body weight. These observations are in line with Ito et al. (2007) who reported hyperlipidemia, obesity, and insulin resistance after the administration of HFD for 10 weeks. Similarly, Bambha et al. (2014) demonstrated that HFD- induced increment in body weight, insulin resistance, inflammatory response, and oxidative burden. Some studies reported activation of PPARγ following HFD administration, resulting in increased food intake and adipose tissue accumulation (Larsen et al., 2003; Sikder et al., 2018).
Table 5: HPLC analysis of total phenolic, flavonoids, and vitamin C contents (μg/ml) of EEGS.
| Vanillin | Salicylic acid | Chloro-genic acid | Cinnamic acid | Syringic acid | Ferulic acid | P-Coumaric acid | Gallic acid | Caffeic acid | Total phenolic | ||||||||
| 5.71 | 6.09 | 26.38 | 28.73 | 29.55 | 34.12 | 36.52 | 36.94 | 39.64 | |||||||||
| Hesperetin | Apigenin | Kaempferol | Rosmarinic acid | Quercitrin | Quercetin | Catechins | Rutin | Total flavonoid | |||||||||
| 1.47 | 1.53 | 1.63 | 1.92 | 2.24 | 2.58 | 3.26 | 3.84 | ||||||||||
| 1.265 | Vitamin C | ||||||||||||||||
Feeding HFD for 12 weeks of the damaged liver in this study, and this can clearly be evidenced by a higher serum ALT and AST activity. This is not surprising observation because similar consistency in results has been observed previously by others (Bugianesi et al., 2005; Yadav et al., 2009). There are studies that evidenced HFD induced increment in hepatic TAG, FFA accumulation, fatty acid synthesis, and oxidation. Such variation in the normal physiological processes resulted in hepatic oxidative damage, NAFLD, and NASH leading to enzyme leakage in the blood (Rolo et al., 2012; Than and Newsome, 2015). Similar to our data, Sanches et al. (2015) observed an occurrence of NASH after 12 weeks by feeding on HFD. Administration of EEGS for six weeks, following feeding on HFD, restored liver enzyme activity. This supports previous research observations (Althnaian., 2014) where hepatoprotective potency of GC was observed following treatment with 3g and 6g per kg diet of rats. These rates were fed high cholesterol diet, and the addition of GC in the feed resulted in decreased ALT and AST activity.
HFD-induced hyperlipidemia was evidenced by the rise in TAG, LDL-C, VLDL-C, TC, and low HDL-C content along with an upregulation in the rate of expression of HMGCR in the current study. These observations are consistent with those reported previously by others (Jiang et al., 2018; Feng et al., 2019). Hypercholesterolemia may be caused by an increased uptake of exogenous cholesterol and increased esterification of free fatty acids. HMGCR is the regulator for cholesterol synthesis; however, similar to previous studies, we observed an upregulation in the rate of expression of this particular gene upon administration of HFD in rats (Jiang et al., 2018; Naik et al., 2018). Hyper-triacylglyceridemia may be due to inhibition of 7a-hydroxylase activity (Beigneux et al., 2002). A reduction in HDL-C level is attributed to a low lecithin-cholesterol acyltransferase (LCAT) activity that facilitates cholesterol uptake from peripheral tissues to HDL in the liver (Kunnen and Van Eck, 2012).
In the current study, HFD- induced hyperlipidemia was significantly reduced by EEGS, which may be related to increased activity of LCAT enhancing HDL-C formation (Shukla et al., 2015). Further to this, quercetin is one of the flavonoids that downregulate lipogenic genes in mice and in vitro models of NAFLD (Pisonero-Vaquero et al., 2015; Wang et al., 2016). Rutin has also been reported to inhibit lipogenesis and facilitate fatty acid metabolism (Liu et al., 2017). With these observations, we ascertain that EEGS-induced hypolipidemic effect may be attributed to flavonoids and phenols content, and this is in agreement with the previous study (Subramania et al., 2017).
Our data proposed that, compared to control, HFD induce hepatic oxidative burden, and this can be evidenced by a decline in hepatic GSH concentration, SOD, CAT activity and an increase in MDA and serum nitric oxide content. This corresponds to previous observations reported by others (Bambha et al., 2014; Than and Newsome, 2015). Glutathione peroxidase reduced hydrogen peroxide and protected from the lipid peroxidation process in the presence of glutathione (Lubos et al., 2011). Manganese-dependent superoxide dismutase prevented mitochondrial oxidative stress in the liver (Mansouri et al., 2010). Fatty acid oxidation is a vital source for ROS generation in fatty livers that attack polyunsaturated fatty acids resulting in lipid peroxidation (Valenzuela and Videla, 2011). Cholesterol is auto-oxidized to oxysterols- exerted apoptosis in NASH (Subramanian et al., 2011). Following high-fat diet feeding, a higher activity of hepatic iNOS are reported that stimulate higher production of hepatic NO (Forstermann and Sessa, 2012; Castiglione et al., 2018; Mohamaden et al., 2019). Hepatic oxidative stress has been associated with a reduced antioxidant capacity (Bahr et al., 2019b). Therefore, GSH concentration, SOD, and CAT are believed to scavenge ROS along with MDA and nitric oxide production.
Our data showed that administration of 400mg/kg EEGS along with HFD for six weeks evoked antioxidant activity, and this was indicated by the upregulation of hepatic SOD, CAT activity and GSH concentration along with a significant decrease in MDA and nitric oxide content. These findings are consistent with those reported previously by others (Umesha and Naidu, 2015; Questi et al., 2016; Al-Sheddi et al., 2016). Added to this, phenolic compounds and flavonoids possess hydroxyl groups that contribute to their antioxidant potential (Bendary et al., 2013; Panche et al., 2016). Gallic acid is one of a phenolic compound that has been reported to improve liver GSH level, GSH reductase, GSH peroxidase, and GSH S- transferase activity, thereby preventing NAFLD and NASH in HFD-fed rats (Hsu and Yen., 2007). Compared to vitamin C supplements, deficiency of Vitamin C for 57 days in gulo−/− mice induced increase in the protein carbonyls, and a decrease in catalase expression (Amano et al., 2013). Vitamin C scavenge free radicals and facilitate reversion of hyperlipidemia, whereas its deficiency can develop NAFLD (Ipsen et al., 2014). Gao et al. (2013) reported anti-inflammatory and antioxidant properties of rutin in the HFD-induced obesity model. Hydrogen peroxide produces hydroxyl radical-induced lipid peroxidation (Saed-Moucheshi et al., 2014). Hence, the prevention of H2O2 production by EEGS antioxidants highlights its biological importance that was further confirmed with our DPPH assay.
The upregulation of vascular endothelial growth factor A (VEGF) in our study is in agreement with previous literature (Forstermann and Sessa, 2012; Castiglione et al., 2018). Coulon et al. (2012) explained the role of VEGF in angiogenesis and progression of NAFLD. Kim et al. (2010) proposed angiogenesis dependent adipose tissue growth that requires VEGF. The upregulation of VEGF expression was noted in hepatic fibrosis in clinical (Paternostro et al., 2010) and experimental studies (Corpechot et al., 2002; Huang et al., 2013; Lin et al., 2014). EEGS treatment in HFD-treated rats exhibited significant suppression in expression of VEGF. This is consistent with previous observations Surapaneni et al. (2015) who proved that quercetin downregulates VEGF expression more than hydroxy citric acid and pioglitazone in NASH rats model. Domitrović et al. (2013) revealed the antioxidant, and antifibrotic prospect of rosmarinic acid in acute hepatic intoxication. Taking altogether, the findings conferred thesafe hepato-protective dose of EEGS along with its antioxidant potential in vivo, and in vitro.
Conclusion
The study concludes that daily administration of EEGS 400mg/kg b.w for six weeks is safe and possess hepatoprotective, antioxidant, hypolipidemic, anti-obesity, anti-angiogenic, and anti- steatosis effects. Different constituents of EEGS such as phenolic, flavonoid, vitamin C content have antioxidant properties that do facilitate encountering the negative consequences of HFD-induced NAFLD.
Authors Contribution
All authors contributed equally.
Conflict of interest
The authors have declared no conflict of interest.
References