Journal of Animal Health and Production
Research Article
The Effect of Some Anti-inflammatory Drugs on Some Clinicopathological Parameters in Barki Sheep
Asmaa Abdallah Darwish1, Mohamed Fahmy Eldakroury2*
1Animal and Poultry Health Department, Animal and Poultry Health Division, Desert Research Center, Cairo, Egypt; 2Department of Pharmacology, Faculty of Veterinary Medicine, Matrouh University, Egypt.
Abstract | Anti-inflammatory drugs either steroidal or non-steroidal have a dual effect on the animal health. Although they enhance healing process, they seriously affect the clinicopathological parameters. This study aimed to compare the adverse effects of three common anti-inflammatory drugs on some hemato-biochemical parameters and spot the light on the importance of Matrix metalloproteinase-2, -9 (MMP-2, MMP-9) in monitoring the anti-inflammatory action of these drugs. Thirty ewes (1 year, 45 Kg) were randomly divided into 3 equal groups, 1st group (DG) was treated with dexamethasone, 2nd group (DIG) received dipyrone while Meloxicam was given to ewes of the 3rd group (MG). Blood samples were taken on 0, 3, 7, 15 and 21 day post treatment. It was observed that DG showed a significant (P< 0.05) leukocytosis, neutrophilia, eosinophilia, lymphocytopenia at 3rd and 7th day accompanied by hypernatremia at the 3rd day compared to DIG and MG. A significant (P< 0.05) decrease was recorded in MMP-2 and MMP-9 in all treated groups. MMP-2 and MMP-9 returned to their baseline values at 15th and 21st day respectively. The adverse effect of dexamethasone on the leukocytes and serum Na level is more prominent than dipyrone and meloxicam and lasts longer for leukocytes. MMPs may be useful in following up the anti-inflammatory action of dexamethasone, dipyrone and meloxicam.
Keywords | Dexamethasone, Dipyrone, Meloxicam, Clinicopathological parameters, Matrix metalloproteinase-2, -9
Received | March 29, 2020; Accepted | May 27, 2020; Published | June 05, 2020
*Correspondence | Mohamed Fahmy Eldakroury, Department of Pharmacology, Faculty of Veterinary Medicine, Matrouh University, Egypt; Email: Mohamed_542000@yahoo.com
Citation | Darwish AA, Eldakroury MF (2020). The effect of some anti-inflammatory drugs on some clinicopathological parameters in Barki sheep. J. Anim. Health Prod. 8(3): 93-100.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/8.3.93.100
ISSN | 2308-2801
Copyright © 2020 Darwish and Eldakroury. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Anti-inflammatory drugs are a basic part of different therapeutic strategies in both human as well as animal medicine. They are extensively used in treatment of various disorders such as inflammation, autoimmune diseases, dystocia, postoperative complications, heart diseases and malignancy (Mistry et al., 2016; Amin et al., 2017; Neamah et al., 2019). Beside its role in inflammatory resolution and healing enhancement, it has analgesic-antipyretic effect. They are classified into two large categories steroidal and non-steroidal. Recently, different commercial products of anti-inflammatory drugs either steroidal or non-steroidal are available in the veterinary field, without sufficient information about their side effects on the haemato-biochemical parameters in sheep (Al-Rekabi et al., 2009; AbdElazem et al., 2015; Burmańczuk et al., 2016; El-Sayed et al., 2017).
Matrix metalloproteinases (MMPs) are sensitive inflammatory markers, usually activated during inflammation under the effect of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) to modulate extracellular matrix turnover and cleavage. Basically, they are proteolytic enzymes, among them MMP-2 and MMP-9 are the most famous enzymes. They named also gelatinases, as they are fundamentally concerned with gelatin degradation. They are embedded in vital physiological process as angiogenesis, apoptosis, blood vessels basement membrane degradation (facilitating cellular infiltration), stimulation and suppression of inflammatory mediators activity. Their blood concentrations are regulated by tissue inhibitor of metalloproteinases (TIMPs). Any imbalance between MMPs and TIMP leads to appearance of wide section of pathological conditions particularly inflammation and tumor. At the last few decades, MMPs were introduced as therapeutic target in different animal and human diseases and were recommended as a follow up tool for the effect of many drugs (Jabłonska-Trypucet al., 2016; Fields, 2019).
Hence, this study aimed to compare side effects of three anti-inflammatory drugs (dexamethasone, dipyrone, meloxicam), commonly used in the veterinary practice on some clinicopathological parameters in healthy Barki sheep with especial reference to their effect on MMP-2 and MMP-9 activity.
MATERIALS AND METHODS
Animals
Thirty Barki non-pregnant apparently healthy ewes (aged 1 year- average weight about 45 Kg) were used in this study. They were kept for a month in a closed farm related to Sustainable Development Center for Matrouh Resources (SDCMR), Desert Research Center (DRC), Egypt. They were fed mainly on concentrates (14-16% protein), consist of 50% maize, 20-30% wheat bran, 10-15 soya bean meal, 10-15% cotton seed cake, 1% minerals and lime stone (900-1100 g/head/day) and hay (350-600 g/head/day). All animal treatment and handling ethics were taken in consideration in this work.
The animals were divided equally into the following groups:
Dexamthasone group (DG): ten ewes were injected intramuscularly with dexamethasone 2% sodium phosphate (1.5 ml/50 kg), a product of ArabcoMed company, Egypt.
Dipyrone group (DIG): ten ewes were injected with dipyrone 50% (Analgin®) intramuscularly (0.5 ml/10kg), a product of UCCMA, Egypt.
Meloxicam group® (MG): ten ewes were injected with Meloxicam (Movac®) 2% intramuscularly (1ml/40kg), a product of ADWIA Company, Egypt.
All dosages and administration method were recommended by the manufacturing companies.
Sample collection and analysis
Blood samples were collected via jugular vein puncture from the thirty ewes at 0, 3, 7, 15 and 21 day post treatment. Each blood sample was divided into two portions: 10% K2EDTA salt solution was added to the 1st portion to stop the coagulation process and this portion was used immediately for assessment of hematological parameters in the three studied groups manually following Feldman et al. (2000) method. The blood in the second portion was centrifuged at 37 ºC 3000 r.p.m. for 20 minutes to get serum for other subsequent biochemical examination.
According to the manual instructions, the estimated serum levels of the biochemical parameters were determined spectrophotometrically by using Biodiagnostic company® commercial kits, Cairo, Egypt. While, MMPs serum concentrations were evaluated by ELISA technique using Cloud- ELISA kits of Clone Corp company®, Huston, USA.
Statistical analysis
Measured parameters were presented as mean ± standard deviation (SD). SPSS program version 24 was used to compare between means of different statistical parameters (two-way ANOVA test) and estimate the post hoc differences between means (a multiple comparison Tukey`s HSD test). A difference was considerable significant at P< 0.05.
RESULTS
Hematological parameters
Table 1 showed a significant (P< 0.05) decline in red blood cell count (RBCs), hemoglobin concentration (Hb), packed cell volume (PCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) in the three studied groups (compared to 0 day) reaching its lowest values at the 3rd day for RBCs, Hb and PCV and at 7th day for MCH and MCHC. While, mean corpuscular volume (MCV) values (compared to 0 day) significantly (P< 0.05) increased at the 3rd day then significantly (P< 0.05) decreased at 7th day. All red blood cell parameters and indices returned to the baseline values at the 15th day.
On the other hand, a significant (P< 0.05) neutrophilic eosinophilic leukocytosis as well as a significant (P< 0.05) lymphocytopenia (compared to 0 day) were depicted in DG only. The peaks of these leukocytosis and lymphocytopenia were at the 3rd day then returned to their normal values at the 15th day. On contrast, both of DIG and MG showed a significant (P< 0.05) mild decreases in TLC and neutrophils count (compared to 0 day) at the 3rd day then rapidly backed to their original values at the 7th day. Non-significant changes were obtained in monocytes and basophils count in all treated groups and in lymphocytes and eosinophil count in DIG and MG (Table 1).
The comparison between the effect of the three drugs on the hematological parameters revealed a significant (P< 0.05) leukocytosis, neutrophilia, eosinophilia and lymphocytopenia in DG when compared to DIG, MG at the 3rd and 7th day (Table 1).
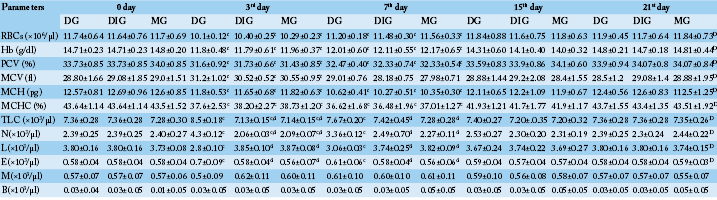
Table 1: Comparison between the effect of the studied anti-inflammatory drugs on some hematological parameters (N=10). Values are means ± SD.
D (significant along the experiment duration), d (significant with DG), c (significant with 0 day) considered statistically significant at P<0.05. DG, Dexamthasone group; DIG, Dipyrone group; MG, Meloxicam group. RBCs, Red blood cell count; Hb, Hemoglobin concentration; PCV, Packed cell volume; MCV, Mean corpuscular volume; MCH, Mean corpuscular hemoglobin; MCHC, Mean corpuscular hemoglobin concentration; TLC, Total leukocytic count; N, Neutrophils; L, Lymphocytes; E, Eosinophils; M, Monocytes; B, Basophils.
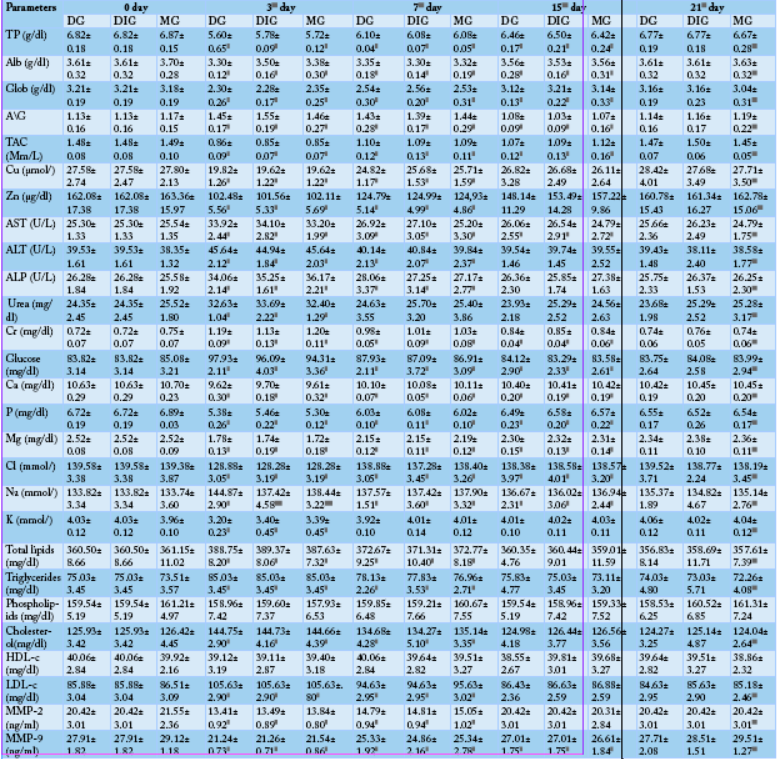
Table 2: Comparison between the effect of the studied anti-inflammatory drugs on some biochemical parameters (N=10). Values are means ± SD.
D (significant along the experiment duration), d (significant with DG), c (significant with 0 day values) considered statistically significant at P<0.05. DG, Dexamthasone group; DIG, Dipyrone group; MG, Meloxicam group. TP, Total protein; Alb, Albumin; Glob, Globulin; A/G, Albumin/Globulin ratio; TAC, Total antioxidant capacity; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; ALP, Alkaline phosphatase; Cr, Creatinine; HDL-c, HDL-cholesterol; LDL-c, LDL-cholesterol; MMP-2, Matrix metalloproteinase-2; MMP-9, Matrix metalloproteinase-9.
Biochemical parameters
The data presented in Table 2 revealed a significant (P< 0.05) reduction in total protein (TP), albumin (Alb), globulin (Glob), total antioxidant capacity (TAC), Zn, Cu, Ca, P, Mg, K, Cl, MMP-2 and MMP-9 levels of DG, DIG and MG when compared to the 0 day. The lowest values of these parameters for the three groups were recorded at the 3rd day then they progressively increased till approaching concentrations close to their initial concentrations (0 day concentrations) at 7thday (K), 15th day (Zn, Cu, MMP-2) and 21stday (TP, Alb, Glob, TAC, Ca, P, Mg, Cl, MMP-9). Contrariwise, A/G ratio (compared to the 0 day) significantly (P< 0.05) elevated at the 3rd and 7th day and significantly (P< 0.05) diminished at the 15th day and backed to its beginning ratio at the 21st day.
On the opposite side, a significant (P< 0.05) increment was determined in the serum levels of glucose, liver enzymes (AST, ALT, ALP), kidney function tests (blood urea, Creatinine (Cr)), total lipids, triglycerides, cholesterol, LDL-cholesterol (LDL-c) and Na in DG, DIG and MGcompared to 0 day. They reached their top values at the 3rd day then they gradually declined reaching their primary levels at 7th day (blood urea), 15th day (ALT, ALP, total lipids, triglycerides, cholesterol, LDL-cholesterol) or 21st day (glucose, AST, Cr, Na). Non-significant changes were noticed in the phospholipids or HDL-cholesterol (HDL-c) concentrations in the three studied group along the research (Table 2).
By comparing the biochemical parameters of the three studied groups, a significant hypernatremia (P< 0.05) was displayed between DG and the other two groups at the 3rd day then quickly recovered at 7th day. While, all the other estimated biochemical parameters non-significantly changed between the three groups along the study (Table 2).
DISCUSSION
Dexamethasone or synthetic glucocorticoids is one of the most common steroidal anti-inflammatory drug used in veterinary field. Dipyrone and meloxicam are non-steroidal drugs also extensively used in the veterinary medicine. In the current work we tried to focus on their side effects on some hematological as well as biochemical parameters. Concerning the hematological parameters, the present study as well as prior studies (Al-Rekabi et al., 2009; Abd Elazem et al., 2015; Burmańczuk et al., 2016; El-Sayed et al., 2017) cleared that dexamethasone, dipyrone and meloxicam induced a mild macrocytic hypochromic anemia which performed in this work at the 3rd day by the distinguished decrease in RBCs, Hb, PCV, MCH, MCHC and the prominent increase in MCV. Whereas the free radicals, usually liberated, attack RBCs membrane phospholipids causing its oxidation and destruction. Besides that, they inhibit bone marrow erythropoiesis by hindering erythropoietin secretion due to oxidative renal injury (Sanchez et al., 2002; Costa et al., 2006; Amin et al., 2017; Neamah et al., 2019). Lipid metabolism impairment commonly associated with dexamethasone administration is more specific cause for this anemia in DG, as the improper lipid bioavailability suppressed RBCs generation (Zingariello et al., 2019). On the other side, the elevated MCV numbers at 3rd day then their decrease at 7th day referred to a compatible bone marrow response. By the 7th day, the red blood cell parameters started to increase towards their normal values but both MCH and MCHC continued their diminishing and the type of anemia transformed to normocytic hypochromic anemia. Finally, at the 15th day all the parameters were corrected and anemia disappeared in all groups. Similarly, a fine leukocytopenia and neutropenia was depicted in DIG and MG at the 3rd day. This leukocytopenia and neutropenia was mainly attributed to the suppressive effect of both diyprone and meloxicam on the pro-inflammatory cytokines activity which are the basic regulator for granulocytes proliferation, differentiation, maturation, release in the circulation and migration (Gozzoli et al., 2004; Grauw et al., 2009; Al-Rekabi et al., 2009). In addition, previous authors mentioned the toxic effect of diyprone on circulating neutrophils and myeloid progenitor cells in the bone marrow, through stimulating of immune complexes (anti-neutrophils) formation and subsequently increasing neutrophils lysis (Burmańczuk et al., 2016; Machado-Alba et al., 2018). Unlike DIG and MG, the data of DG displayed an observable increase in TLC, neutrophils and eosinophils accompanied with a prominent lymphocytopenia at the 3rd day. Actually, dexamethasone has a simple contribution to the bone marrow granulopoiesis and the outstanding leukocytosis and lymphocytopenia in DG returned to the migratory effect of dexamethasone on PMNs from marginated pool to the circulating pool on the expanse of the lymphocytes but it also has an immunosuppressive effect as diyprone and meloxicam as it decreases the neutrophilic phagocytic activity (Nakagawa et al., 1998; Narimane et al., 2017; Corum et al., 2016). The reappearance of the normal leukogram in DIG and MG was faster than DG, because the leukocytopenia and neutropenia degree in DIG and MG was lower than the leukocytosis and neutrophilia degree in DG.
Regarding the protein profile, a remarkable hypoglobulinemia, slight hypoalbuinemia and dependent hypoproteinemia and fluctuated A/G ratio were noted in the three studied groups at the 3rd day. These alterations were chiefly assigned to the inhibitory effect of the three studied drugs on the pro-inflammatory cytokines and the consequently decreased immunoglobulins production (γ-globulin) as well as acute phase proteins (α, β-globulin) (Al-Rekabi et al., 2009; Abd Elazem et al., 2015; Gozzoli et al., 2004; Khan and Mishra, 2011). Meanwhile, the outstanding oxidative stress detected in the present research as well as in former studies may be a reasonable cause for the distinguished hypoalbuminemia here, as albumin is known to be a potent antioxidant and logically was consumed to neutralize this oxidative stress (Sanchez et al., 2002; Turgay et al., 2012; Mikryakov et al., 2014; Amin et al., 2017; Neamah et al., 2019). Obviously, the late achievement of the protein profile component for their baseline values at the 21st day confirmed the immunosuppressive effect of the studied three drugs.
With respect to the total antioxidant capacity, a marked decline was observed in the three studied groups at 3rd day. Whereas the generated free radicals associated with the three drugs metabolism usually resulted in a noticeable inhibition in the different endogenous antioxidants activities and a subsequent appearance of the oxidative stress (Liu et al., 2012; Amin et al., 2017; El-Sayed et al., 2017; Neamah et al., 2019). Interestingly, the decreased levels of Zn and Cu demonstrated in the three groups at the 3rd day (which are vital co-factor in antioxidant enzymes composition) pointed to a serious trial from the body to control this oxidative stress by using both elements in synthesize of more antioxidant enzymes (Liu et al., 2012; Amin et al., 2017; El-Sayed et al., 2017; Neamah et al., 2019). Rationally, the oxidative stress reported in this paper is extremely involved in the hepatic and renal cell damage leading to the noted increase of AST, ALT, ALP, creatinine and urea in the three studied groups (Abd Elazem et al., 2015; Amin et al., 2017; El-Sayed et al., 2017; Neamah et al., 2019). In turn, the glucose uptake from the blood by the hepatocytes was inhibited causing the described hyperglycemia in the three studied groups at the 3rd day (Corum et al., 2016; El-Sayed et al., 2017). Additionally, dexamethasone mostly interferes with insulin secretion from pancreatic islets and increase peripheral insulin resistance thus sharing in the spotted hyperglycemia in DG (Corum et al., 2016). In the same way, the impaired kidney functions are widely contributed to the altered minerals and electrolytes levels detected in the three groups at the 3rd day. Whereas, kidneys are the major regulator for minerals and electrolytes excretion and restoring (Abd Elazem et al., 2015; Amin et al., 2017; Neamah et al., 2019). Certainly, the mechanism of action of dipyrone and meloxicam as non-steroidal anti-inflammatory drugs also took a part in the reported hypocalcaemia, hypophosphatemia, hypomagnesaemia, hypokalemia and hypocholidemia in DIG and MG at the 3rd day. As they suppress COX-1 activity resulting in serious gastrointestinal mucosal injury and hinder minerals and electrolytes absorption (Amin et al., 2017; Neamah et al., 2019). On the other hand, the selective action of dexamethasone on Na retention through natriuretic diuresis reduction possibly explains the recorded hypernatremia in DG in relation with the other two groups at the 3rd day (Mistry et al., 2016).
Another outcome for the above-mentioned oxidative stress, is the obtained LDL-hypercholesterolemia and linked hypercholesterolemia in the three studied groups at 3rd day. Whereas the increased lipid peroxidation related to this oxidative stress usually associated with an increase in the circulating LDL/total cholesterol levels. While, the hypertriglyceridemia noticed in the present research in all groups at 3rd day indicated an increased lipolysis and may referred to a considerable drop in the gastrointestinal fat absorption. Besides that, the dexamethasone inhibitory effect on insulin secretion is strongly involved in both hypercholesterolemia as well as hypertriglyceridemia. Reasonably, these hypercholesterolemia and hypertriglyceridemia led to the outstanding hyperlipidemia in the three groups at the 3rd day (Liu et al., 2012; Amin et al., 2017; Dolatabadi and Mahboubi, 2015; Neamah et al., 2019).
Concerning MMP-2 and MMP-9 activity in the present report, their decline in the three studied groups at the 3rd days, returned to the suppressive action of dexamethasone, diyprone, and meloxicam on the pro-inflammatory cytokines which are the main MMPs secretion stimulators, especially TNF-α and IL-1β (Ranisford et al., 1997; El Azab et al., 2002; Al-Rekabi et al., 2009; Grauw et al., 2009; Cui et al., 2015). These findings approved the immunosuppressive effects of the studied drugs. The above-mentioned hypozincemia and hypocalcaemia in the three studied groups may also participated in MMPs diminishing activity as both of Zn and Ca are necessary cofactors for MMPs synthesis and activation (Al-Rekabi et al., 2009; Grauw et al., 2009; Cui et al., 2015). Interestingly, the gradual increase or decrease in all evaluated parameters levels till approaching their primary values at 7th, 15th or 21st day, cleared that these side effects are transient and approved the body ability to overcome them.
Finally, it can be concluded that dexamethasone, diyprone and meloxicam administration have several adverse effects on the estimated hematological and biochemical parameters. These effects are temporary and reversible and mostly connected with the oxidative stress resulted from the drug metabolism. Dexamethasone effect on the leukocytes and Na levels is more observable than dipyrone and meloxicam and extends longer for leukocytes only. MMPs may be useful indicators for the anti-inflammatory effect of dexamethasone, diyprone and meloxicam.
Acknowledgements
Members of animal husbandry unit, Sustainable Development Center for Matrouh Resources (SDCMR), Desert Research Center (DRC), Egypt.
Authors Contribution
All authors contributed equally.
Conflict of interest
The authors have declared no conflict of interest.
References






