Journal of Animal Health and Production
Research Article
Cellular Responses of Mouse Vaginal Cells Treated with Korean Native Plant Extracts
Gwang Joo Jeon1,2,*, Sanghwan Kim2, Jihye Lee2, Hyemyoung Jang1, Tae Eun Kim3, Inkwon Han4, Youngik Ko5, Yoon Dong Choi6, Duk Ho Kim7
1Department of Biotechnology, Hankyong National University, Ansung, Korea; 2Genomic Informatics Center, Hankyong National University, Ansung, Korea; 3Eun Skin Clinic, Seoul, Korea; 4KMP Health Care Seoul Clinic, Seoul, Korea; 5Michaewon Clinic, Korea; 6Seoul Hoseo Occupational Training College, Seoul, Korea; 7DK Costech Co., Ltd. Korea.
Abstract | In current experiment, three Korean native plants (Dendropanax morbifera LEV (DM), Astragalus membranaceus (AM) and Aralia elata (AE) in three different combination groups (D1=DM only, D2=DM+AM, D3=DM+AE) were tested for their effects on epithelial cells of mouse vagina, as an alternative model for human cells. A total of 100 ICR mice (14 weeks old) were equally divided into four groups. One group served as a control while the other groups were treated with methanolic extract of plants. One ml solution of either of plant extract (D1, D2 or D3) was inserted into mouse vagina at depth of 1 mm using a round tip needle. After 24 h of treatment, the mouse vaginal endothelial cells were analyzed for expression of various proteins like PCNA, BCL-2 and caspase-3. The immunohistochemistry, western blot and microscopic results showed that PCNA protein localization detected in epithelium cells of mouse vagina, furthermore, PCNA protein was highly detected in basal and mucosa epithelium cells in D3 group compared to group D1 and D2. Cell survival factors were highly expressed in mucosa epithelium cells of D3 group compared to other treatment groups (D1 and D2) but apoptosis factor was more expressed in D1 and D2 than the D3 groups. Western blot analysis showed that protein detection of cell survival PCNA is highly expressed compare to β-actin, BCL2, and Casp-3. Cellular responses of vaginal epithelial cells demonstrated hypertrophy, hyperplasia and other patterns of cell adaptation. It was concluded that external stimuli of DM alone and its’ combination with AM and AE showed hypertrophy, hyperplasia and other related protein expressions depending upon the plant combinations.
Keywords | PCNA, BCL-2, Caspase-3, Korea native plants
Received | September 20, 2019; Accepted | November 02, 2019; Published | February 20, 2020
*Correspondence | Gwang Joo Jeon, Department of Biotechnology, Genomic Informatics Center, Hankyong National University, Ansung 17579, Korea; Email: Jeon5894@gmail.com
Citation | Jeon GJ, Kim S, Lee J, Jang H, Kim TE, Han I, Ko Y, Choi YD, Kim DH (2020). Cellular responses of mouse vaginal cells treated with korean native plant extracts. J. Anim. Health Prod. 8(1): 27-31.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/8.1.27.31
ISSN | 2308-2801
Copyright © 2019 Jeon et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Proliferating Cell Nuclear Antigen (PCNA), one of the cell activating factors, is a major marker for cell survival (Liu et al., 2018). The expression of PCNA in uterus and mammary gland by the treatment of isoflavone genistein has been previously reported (Rimoldi et al., 2007; Al-Snafi 2018; IIhsan et al., 2019). Glover et al. (2017) reported that PCNA is a major protein influencing DNA replication, repair of DNA damage, chromatin structure maintenance, chromosome segregation and cell-cycle progression in cancerous cells. Ito et al. (2015) studied the effect of Semaphorin 4D on vaginal epithelial cell apoptosis and suggested that the expression of Caspase-3 is highly influenced by this agent.
Dendropanax morbifera Levillis is a native Korean plant and is mostly growing in southern parts of Korea (Youn et al., 2019). Koreans have strong belief on medicinal plants particularly the health effects of this plant. The effects of D. morbifera are numerously published for their medicinal purposes and other daily applications as a healthy food (Song et al., 2019). Kim et al. (2017) reviewed various effects of this plant as anti-cancer and anti-oxidant especially in postmenopausal period. Seo et al. (2016) reported positive effects of D. morbifera on the excretion of heavy metals and improving the antioxidant effects. Park et al. (2014) reported that rutin, one of the main components in this plant has a therapeutic potential for the treatment of neurodegenerative diseases associated with oxidative stress and inhibition of p38 MAPK signaling to protect cell injury. The medicinal effects of Astragalus membranaceus was thoroughly reviewed by Auyeung et al. (2016). Like other medicinal herbs, this plant is also well known for its anticancer effect. Aralia elata has been used as a medicinal herb and its effects are well known for diabetes and gastritis (Asnaashari et al. 2019).
The main causes of cell injury are mainly due to the external stimuli of physiological and pathological factors. In reality, it is hard to find out the process of morphological changes in living tissues and organs. Morphological patterns of epithelial cells of mouse vagina could be different depending upon the estrous cycles and the expression of proteins differs in different stages of estrus most abundant during pre-estrus (Li et al. 2019). A simple criterion for reversible or irreversible state depends on whether the injured cells are adaptable to the stimuli and return to the normal state. The purpose of this paper was to find the effects of Korean native medicinal plants viz., D. morbifera, A, membranaceus and A. elata on cellular responses of mouse vagina endothelial cells.
Materials and Methods
In this study, ICR mice were provided by Experimental Lab of Seoul Hoseo Occupational Training College, Korea. The protocol was approved by Seoul Hoseo Institutional Animal Care and Use Committee (HS-2018- 2).
Experimental Animals
A total of 100 female mice (14 weeks old) during their estrus phase were divided into four equal groups. One group served as a control (and treated with normal saline) while the other groups were treated with methanolic extract of D. morbifera (D1), D. morbifera + A. membranaceus (D2) and D. morbifera + A. elata (D3). The methanolic extract of each plant was prepared by standard protocol (Kumosani et al., 2018). One ml solution was inserted into mouse vagina at depth of 1 mm using a round tip needle. All the mouse were euthanized after 24 h and uterus and ovaries were removed.
Western Blot Analysis
The preparation of samples and procedure were followed to the method of Kim et al. (2012). Briefly, from each sample, 30 µg of protein was separated and analyzed by SDS-PAGE on a 13% SDS-polyacrylamide gel and was transferred to an Immuno-Blot PVDF membrane (Bio-Rad, CA, USA). Each membrane for each sample was blocked using blocking buffer (5% non-fat dry milk) overnight at 4°C. Then, the membrane was washed once for 10 min with washing buffer (TBST: 0.1% Tween 20, 50 mM Tris-HCl (pH 7.6), 200 mM NaCl). For the analysis, the membrane was incubated for 2 h with anti-rabbit PCNA (Abcam, ab18197, MA, USA), anti-rabbit BCL-2 (Abcam, ab201566, MA, USA), anti-rabbit Caspase-3 (Abcam, ab13847, MA, USA) and anti-mouse β-actin (Abcam, ab20272, MA, USA) as the control. After binding, the membranes was washed 3 times with 1×TBS-T buffer for 15 min each and then, was incubated for 2 h with HRP-conjugated anti-rabbit and anti-mouse secondary antibodies. The detection was made using ECL detection kit (Abcam, ab65623, MA, USA) with 5 min incubation in a dark room. The detection reagent was drained and then, the membrane was exposed to a sheet of diagnostic film in a film cassette for 1 to 30 min.
Sample Processing For Histological Examination
The vagina was cut at the entrance part of vagina, roughly 2 mm in length, soaked into 70% EtOH at 4o C for 24 h. After then, it was washed with 1X PBS and then sequentially with 70, 95, 100% EtOH and finally Xylene (Polysciences, PA, USA) at an interval of every 30 min, sequentially. This process was repeated twice and then fixed with paraffin at 58 o C for 2 h and was left for 24 h. Now, it was kept at 4oC until it was used for the microscopic analysis.
Hematoxylin and Eosin (H&E) Stain
Before staining the samples, the paraffin was removed by soaking each tissue slice (5 µm) sample into Xylin, 100% EtOH, 95% EtOH, 70% EtOH and 50% EtOH for 10 min repeatedly twice and then, it was stained with Hematoxylin (Biosesang, Seongnam, Korea) for 3 min. It was then soaked into distilled water for 5 min. It was again soaked into Acid EtOH (0.5%HCL+70%EtOH) for 12 min and then stained with Eosin (Mutp pure chemicals, Tokyo, Jap) for 30 sec. It was again soaked into the sequential process of Xylene, 100% EtOH, 95% EtOH, 70% EtOH and 50% EtOH for 10 min repeatedly twice. Finally, it was sealed with Permount solution (Fisher, NJ, USA) for 48 h to take photos of microscopic images.
Immunohistochemistry (IHC)
IHC process in this study was followed according to the study of Kim et al. (2012). Immunohistochemistry of proteins associated with cell survival and activation was conducted on 5 µm tissue sections on salinized slides. Paraffin sections were dewaxed with xylene substitute (Polysciences, PA, USA) and were rehydrated in a series of EtOH. Antigen was retrieved by heating at 95°C in 10 mM sodium citrate (pH 6.0). Endogenous peroxidases were then quenched with 0.3% hydrogen peroxide in methanol for 5 min at room temperature. After 3 times of washes in 1×PBS buffer, the slides were blocked in 1% goat serum containing 3% horse serum for 1 h at room temperature. Sections were labeled overnight at 4°C with PCNA, BCL-2 and Caspase-3 (diluted 1:300 with blocking buffer). Washed sections were then incubated with anti-rabbit secondary antibodies and Anti-FITC HRP Conjugate (Takara, Osaka, JPN) (diluted 1:300) for 1 h at room temperature, and then rinsed and incubated with ABC detection kit (Vector, CA, USA) for 10 min. Diaminobenzidine (Vector, CA, USA) was used as a substrate for HRP. Sections were counterstained with PAS reagent and Harris hematoxylin containing 4% acetic acid. Then, tissues were dehydrated, cleared, and covered using Permount solution (Fisher, NJ, USA).
Results
Histological Picture of Mouse Vagina
The histological picture of mouse vagina epithelial cells treated with different medicinal plants are shown in Fig. 1. The morphological patterns are quite different in their responses to different treatments. Especially D. morbifera treatment showed the distinct cell swelling patterns of hypertrophy (D1 and D2) and cell proliferated pattern of hyperplasia (D3). However, for treatment of D1, the epithelial cells in the mouse vagina showed swelling without ruptures of nucleus and plasma membrane. The cellular volume of the vagina epithelial cells treated with D2 was, on average, 9 times larger than the control cells and twice as large as D1. However, the cellular volume of the cells treated with D3 showed no significant difference compared with the untreated control cells but the number of cells and density of cell depositions in the similar area were drastically increased to almost 2 or 3 times larger than the untreated control cells, which implies the accelerated rate of cell proliferation process. The pattern of intact cells were well shown in control and D3 while very loosely intact cells were in D2.
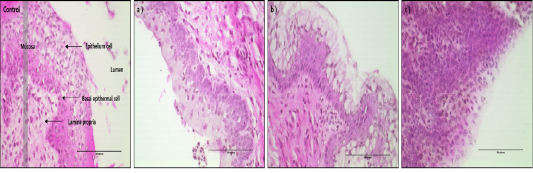
Figure 1: < H&E-stained vaginal tissues after 24hr of treatment with D1, D2 and D3 (magnification, X400). Black bar=100 um. Control group: Normal vaginal epithelium cell of mice, a) D1 treatment group, b) D2 treatment group, c) D3 treatment group.
Western Blot Analysis for Cell Activation Proteins
To investigate the expression of cell survival and apoptosis associated proteins, SDS-PAGE and Western Blot analysis were made in responses to external stimuli of different Korean native plants. Initially SDS-PAGE analysis was made to identify the expression of PCNA, BCL-2 and Caspase-3 through Western blot analysis from which the results were shown in Fig. 2. Protein expression analysis of PCNA, BCL-2 and Casp-3 is shown in Fig. 2. The results of Western blot analysis showed that protein detection of cell survival (PCNA and BCL2) and apoptosis associated factor (Caspase-3) are well expressed (Fig. 2A). Especially, PCNA protein was most highly expressed in D3 treatment group and other products were expressed equally much less than D3. In addition, anti-apoptosis factor of BCL-2 protein levels progressively increased in D1 group to D3 group. As expected, the apoptotic inducer of Caspase-3 was least expressed in D3. Therefore, the protein factor of genes associated with anti-apoptotic and survival were highly detected in D3 treatment (Fig. 2B).
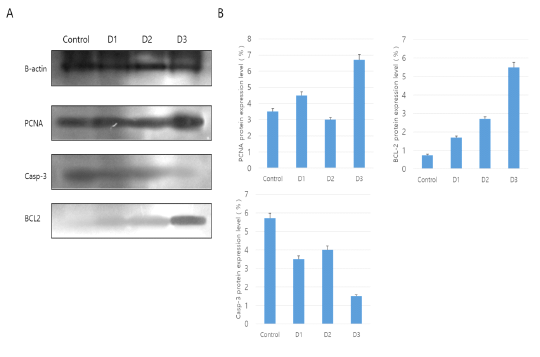
Figure 2: Western blot analysis for PCNA, BCL-2 and Caspase-3 (Casp-3) in vaginal tissue. The mean ± SEM of the treatment groups were normalized according to β-actin (Housekeeping gene) as a standard. A: Western blot analysis, B: Use of Image J program in NIH(Schneider et al.,, 2012).
Immunohistochemistry Analysis
For detection of cell survival and apoptosis related proteins, immunohistochemistry was conducted. Three proteins of PCNA, BCL-2 and Caspase-3 were detected for their expressions in mouse vagina cells (Figure 3). The results of PCNA protein localization analysis was detected in epithelium cells of mouse vagina. Especially, PCNA protein was most highly detected in basal and mucosa epithelium cells in D3 group (Fig. 3A). Among the treatments, most significant expression of PCNA was in D3 and D1 which were higher than control. Least expressed was in D2. For BCL-2, most significantly expression was observed in D3 whereas other treatments showed similar degree of expression (Fig 3B). However, Caspase-3 protein was differently expressed in contrast with survival associated proteins and was least expressed in D3 followed by D1, D2 and control (Fig. 3C). Therefore, cell survival factors were highly expressed in mucosa epithelium cells of D3 compared to other treatment groups but apoptosis factor is more expressed in D1 and D2 than the D3 and control groups.
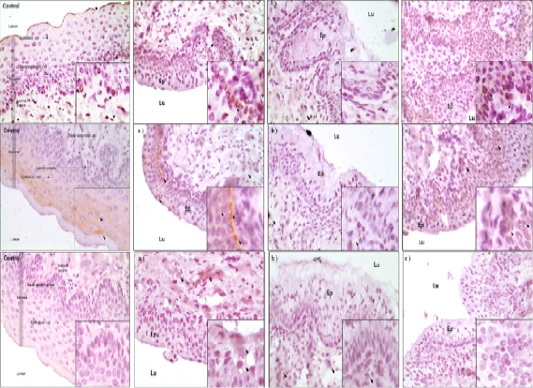
Figure 3: A) IHC analysis of PCNA protein in the vaginal tissue of mice. Black arrows is the protein detected point (magnification X400 in a small box and magnification X100 in a large box). a) D1 treatment group, b) D2 treatment group, c) D3 treatment group. Ep: Epithelium cell well, Lu: Lumen zone; B) IHC analysis of BCL-2 protein in the vaginal tissue of mice. Black arrows is the protein detected point (magnification X400 in a small box and magnification X100 in a large box). a) D1 treatment group, b) D2 treatment group, c) D3 treatment group. Ep: Epithelium cell well, Lu: Lumen zone; C) IHC analysis of BCL-2 protein in the vaginal tissue of mice. Black arrows is the protein detected point (magnification X400 in a small box and magnification X100 in a large box). a) D1 treatment group, b) D2 treatment group, c) D3 treatment group. Ep: Epithelium cell well, Lu: Lumen zone.
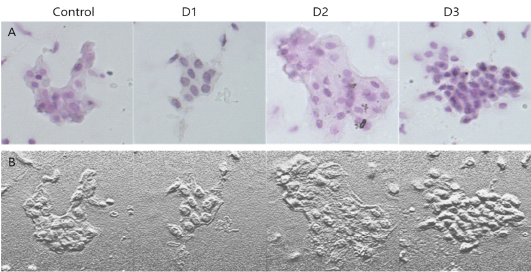
Figure 4: A: H&E staining of mouse vagina epithelial cells treated with D1, D2 and D3. B: Microscopic image analysis of mouse vagina epithelial cells for different treatment groups.
H&E Staining and Microscopic Analysis
The findings of H& E stained mouse vagina cells were shown in Figure 4. In Fig. 4, it was apparent that D3 shows most intact cell patterns with distinct nucleus and cytoplasm than D1 and D2. D2 showed a serious cell rupture pattern by necrosis.
Discussion
Plant extracts showed both hypertrophy and hyperplasia, which implies that the cell tends to control their functionality and structural forms to survive against the different types of injury. The swelling pattern of cells is naturally accepted as one of the theories of cell adaptation patterns. For swelling patterns, we easily presume the influx of water or ionic fluid into the cell. Another possibilities are the multi-production of organelles, proteins, and other biochemical components inside the cell. The phenomena of acute excessive cell proliferation in D3 is of highly interesting. The possible explanation for this phenomena could be that during the cell cycle, before the M stage, D3 would trigger the mitotic event and the cell division would soon initiate. Also, DM inhibits the Caspase-3. Especially PCNA is the cell proliferating and activating factor in S-phase of a cell cycle (Agbana et al., 2019). For the results of cell survival factors expression with treatments of different plants, the most evident results were found in D3 treatment indicating that PCNA activation plays an important role in relation to cell activation during cell remodeling and survival (Kim et al., 2014; Glover et al., 2017).
Argun-Kurum et al. (2019) reported an increase of PCNA that induces the activation of endothelial cells. As studied by Ito et al. (2014), the reduction of cell apoptosis by certain external stimuli influences the cell remodeling and cell activation. As shown in the immunohistochemistry analysis where the antibody to PCNA was used and checked its binding expression; expression of cell growth activator of PCNA was distinctively localized on tissues of mouse vagina cells in comparison with other treatments.
Conclusion
It was found that treatment of D. morbifera alone as well as its’ combination with A. membranaceus and A. elata exhibited hypertrophy, hyperplasia and other related protein expressions in mouse vagina. In addition, combination of D. morbifera and A. elata produced superior results.
Acknowledgements
We highly appreciate Seoul Hoseo Occupational Institute for the use of the animal facility.
Conflict of interest
There is no conflict of interest.
Authors contribution
All authors contributed equally.
References





