Journal of Animal Health and Production
Research Article
Oestrous Response and Conception Rate in Malabari Cross-Bred Goats Following Two Different Oestrus Synchronization Protocols
Sneha Surendra Panicker1, Promod Kanjirakuzhiyil2, Ramachandran Koodathil3, Raji Kanakkaparambil4*
1Department of Animal Reproduction; 2Department of Animal Reproduction; 3Department of Animal Reproduction, Gynaecology & Obstetrics, College of Veterinary & Animal Sciences, Pookode, Wayanad, Kerala 673576, India; 4Department of Veterinary Physiology, College of Veterinary & Animal Sciences, Mannuthy, Kerala 680651, India.
Abstract | The comparative efficacy of two oestrus synchronization protocols using Ovsynch and progestagen (TRIU -C®) was analysed based on the reproductive performance in Malabari cross bred goats. Early onset of oestrus with higher oestrus response was observed in the progestagen group of animals. However, there was no significant difference between the two protocols, on the duration and intensity of oestrus. When diagnosed by ultrasound sonography, the conception rate following the two protocols did not find to vary significantly. Even though, the intensity of oestrus did not vary with the protocols, the intensity was higher (P<0.05) in the conceived group of animals than those failed to conceive. This study suggests that the oestrus intensity has a correlation to the conception rate in goats.
Keywords | Ovsynch protocol, Progestagen, Duration of oestrus, Intensity of oestrus, Conception rate
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | February 28, 2015; Revised | May 05, 2015; Accepted | May 11, 2015; Published | May 24, 2015
This article is a Part of MVSc thesis of Dr. Sneha Surendra Panicker.
*Correspondence | Raji Kanakkaparambil, College of Veterinary & Animal Sciences, Mannuthy, Kerala, 680651, India; Email: raji@kvasu.ac.in
Citation | Panicker SS, Kanjirakuzhiyil P, Koodathil R, Kanakkaparambil R (2015). Oestrous response and conception rate in Malabari cross-bred goats following two different oestrus synchronization protocols. J. Anim. Health Prod. 3(2): 39-42.
DOI | http://dx.doi.org/10.14737/journal.jahp/2015/3.2.39.42
ISSN | 2308–2801
Copyright © 2015 Panicker et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The reproductive management of goats on a large scale becomes difficult due to various contributing reasons like poor oestrus expression, lack of heat detection techniques. In large flocks, oestrus synchronization and fixed time insemination is useful when oestrus expression is poor or there is lack of time to conduct the same to augment fertility in goats (Rahman et al., 2008).
Oestrus synchronization can be carried out by the conventional methods like alteration in the light exposure period, buck exposure and the use of hormonal treatments. Synthetic Gonadotropin Releasing Hormone (GnRH) preparations, equine chorionic gonadotropin (eCG), human chorionic gonadotropin (hCG), progestagens administered by different routes (oral, injections, vaginal pessaries) and prostaglandin (PGF2α) in different combination and regimen are used for oestrus synchronization in ruminants (Whitley and Jackson, 2004).
Promising synchronization results with Ovsynch and Progestagen regimen have been obtained in cattle and sheep on a large scale (Martinez-Tinajero et al., 2011; Azevedo et al., 2014). Ovsynch protocol involves GnRH- PGF2α -GnRH treatment. The first GnRH injection triggers ovulation of large follicle, if present and also helps for the initiation of new follicular wave. PGF2α injection help for the regression of natural and accessory corpus lutea present in the ovary and the ovulation is timed by the next GnRH injection (Holtz et al., 2008). GnRH with prostaglandin F2α is a commonly used synchronization protocol. In Progestagen protocol, exogenous progestagen is given between GnRH and PGF2α to prevent premature expression of estrus prior to PGF2α (McKinniss et al., 2011).
Even though the results are promising with these protocols, very few works have been reported in goats of tropical region in India. Therefore, this particular study in Malabari cross-bred goats was conducted to examine the effect of two regimens on the synchronization of oestrus in goats of tropical region.
Table 1: Nutritive value of concentrate feed in percent
|
Chemical composition |
Crude protein |
Crude fibre |
Fat |
Calcium |
Acid insoluble ash |
Total ash |
Moisture content |
|
Nutritive value |
17.57 |
36.64 |
2.12 |
1.82 |
3.4 |
11.9 |
6.2 |
MATERIALS AND METHODS
Animals and Feeding
A total of 16 healthy Malabari crossbred multiparous goats aged between 3-4 years and body weight around 20-25 kg were selected for the study. The animals were exposed to natural lighting conditions at latitudes between 11°47’ N & 15°58’ N, where maximum temperature and relative humidity is ranging between 27-29oC and 78-88 mm/Hg respectively during the breeding season. They were fed with a daily ration of 200 g concentrate mixture per animal (Table 1). In addition, free access to green grass and water was made available.
Synchronization Protocols
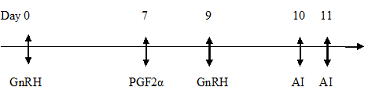
The goats were divided into two groups of eight animals each. Goats belonging to Group I were subjected to oestrus synchronization by using Ovsynch Protocol with first dose of Buserelin injection (0.004mg), GnRH analogue (1ml Receptal®, Intervet, Holland) on day 0 intramuscularly followed by an intramuscular injection of prostaglandin (12.5 mg) Dinoprost (2.5 ml Lutalyse®, Pfizer, Belgium) on 7th day. Forty eight hours after prostaglandin treatment (day 9th), a second dose of Buserelin (0.004mg) was again administered intramuscularly.
Group I (Ovsynch protocol)
Animals in Group II were subjected to the intra-vaginal placement of progesterone device (TRIU-C®, Virbac, India) containing 160 mg of natural progesterone along with intramuscular injection of GnRH (Buserelin,0.004 mg) on the first day of the experiment (day 0). The intravaginal device was retained for seven days and at the time of withdrawal (day 7th) an intramuscular injection of 12.5 mg of Dinoprost, an analogue of PGF2α (2.5 ml Lutalyse®, Pfizer, Belgium) was administered.
Group II (Progestagen-TRIU-C® protocol)
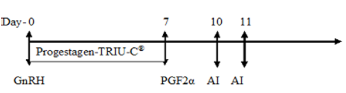
Twenty four hours after the prostaglandin treatment the goats in each group were exposed to apronised bucks for 30 minutes for detection of oestrus and its scoring. The Flehmen’s reaction shown by the teaser buck was primarily observed and the intensity of oestrus signs in does was determined (Table 2). The goats were inseminated with fresh semen 72 hours (day 10th) after the administration of the prostaglandin injection and re-inseminated twenty four hours after the first insemination (day 11th). The does were observed for the onset of oestrus, duration of oestrus and its intensity during this period (Table 3).
Table 2: Oestrus signs and its scoring
|
Behavioural signs |
Score |
||||
|
1 |
Tail wagging |
2 |
|||
|
2 |
Bleating |
1 |
|||
|
3 |
Circling with buck |
1 |
|||
|
4 |
Mounting on other animals |
1 |
|||
|
5 |
Standing to be mounted |
5 |
|||
|
Physiological signs |
Score |
||||
|
6 |
Vulval hyperaemia |
2 |
|||
|
7 |
Vulval oedema |
3 |
|||
|
8 |
Vaginal discharge |
5 |
|||
|
Total |
20 |
||||
Data Analysis
The data were compiled and subjected to statistical analysis as per the method of Snedecor and Cochran (1994). Least squares analysis of variance as described by Harvey (1987) was used to study the effect of synchronization protocols and pregnancy status on various parameters of reproduction. Duncan’s Multiple range test as modified by Kramer (1957) was used to test the differences among least squares means.
RESULTS AND DISCUSSION
In this study, we observed only 75 percent of oestrus response in Malabari Cross-bred goats of group I which have undergone Ovsynch protocol (Table 3 and 4). Holtz (2005) reported the oestrus response to be 100 percent with Ovsynch protocol in Boer cross-bred does during the breeding season. Incidence of silent oestrus in goats has been reported by Greyling and van der Nest (2000). The variation of results in our study could be because of incidence of silent oestrus in our goats as reported by them or due to breed variations and geographical conditions affecting the oestrus.
Table 3: Various parameters of oestrus in group I and II
|
Groups |
Oestrus response(%) |
Oestrus onset interval (h) |
Mean oestrus onset interval (h) |
Duration of oestrus (h) |
Mean duration of oestrus (h) |
Conception rate |
|
Group I (Ovsynch) (n=8) |
75 |
44-56 |
49.92a ± 1.94 |
12-60 |
35.05a ± 4.79 |
58 |
|
Group II (Progestagen TRIU-C®) (n =8) |
10 |
20-36 |
26.00b ± 1.81 |
22-48 |
32.75a ± 4.46 |
50 |
a, b- Means with different superscript vary significantly (P< 0.01); n = No. of animals in each Group.
The oestrus response in group II (Progestagen-TRIU-C®) was 100 percent (Table 3 and 4). The results obtained in the present study were in agreement with the results obtained by Romano (2004) and Godfrey et al. (1997) with different protocols of progestagens. Therefore, these studies suggest that the progestagen (TRIU-C) is more effective than the standard Ovsynch and prostaglandin regimen for the oestrus induction in the goats of tropical region. The effect of PGF2α regimen in the synchronization of oestrus is restricted to only cycling animals as a responsive corpus luteum is required for its action whereas, progesterone promotes the growth of dominant follicle even when a corpus luteum is absent (Wildeus, 1999).
Table 4: Intensity of oestrus in group I (Ovsynch) and Group II (Progestagen) in their oestrus period
|
Group |
No. of animals |
No. of animals responded |
Intensity score range |
Mean intensity score |
|
Group I (Ovsynch) |
8 |
6 |
6-19 |
13.74 a ± 1.57 |
|
Group II (Progestagen-TRIU-C®) |
8 |
8 |
6-20 |
14.37 a ± 1.46 |
a, b- Means with different superscript vary significantly (P< 0.05)
The mean (± S.E.) time taken for the onset of oestrus in Group I (Ovsynch) was 49.92 ± 1.94 hours (Table 3). The result was in close agreement to that of Holtz et al. (2008). The mean (± S.E.) time taken for the onset of oestrus in Group II (Progestagen-TRIU-C®) was 26.00 ± 1.81 hours which was in agreement with Motlomelo et al. (2002) and Romano (2004). The oestrus response in group II (Progestagen-TRIU-C®) was 100 percent. The variation in the time taken for the onset of oestrus with different synchronization protocols have also been reported by Greyling and Van Niekerk (1986). Thus, the difference in the time taken for the onset of oestrus between the two groups in the present study may be due to the differences in the synchronization regimens which are known to influence the onset of oestrus.
The mean (± S.E.) duration of oestrus in Ovsynch group was 35.05 ± 4.79 hours and in progestagen group it was 32.75 ± 4.46 hours (Table 3). The results obtained with Ovsynch protocol in this study were in agreement with those obtained by Holtz et al. (2008). The results obtained by the progestagen protocol were in agreement with that of Holtz et al. (2008) and Motlomelo et al. (2002). The difference in the time taken for the onset of oestrus between the two groups in the present study may be due to the differences in the synchronization regimens The oestrus intensity scores in group I (Ovsynch) ranged from 6-19 with a mean (± S.E.) intensity score of 13.74 ± 1.57. The oestrus intensity score in group II (Progestagen-TRIU-C®) ranged from 6-20 and the mean (± S.E.) intensity score was 14.37 ± 1.46 (Table 4). Though the treatment regimens followed were different, the mean intensity of oestrus was almost similar in these goats of similar agro-climatic region.
The mean (± S.E.) intensity of oestrus (17.87 ± 1.46) scoring in the animals that conceived was significantly higher than those that did not conceive (10.24 ± 1.57) (Table 5). So far, there have been no reports regarding the correlation of the mean intensity scores to the conception rates in goats. However, it is suggestive that the oestrus intensity has a correlation to the conception rate in goats and this warranties further study.
Table 5: Intensity of oestrus in pregnant and non-pregnant animals of both groups (I and II)
|
Intensity score range |
Mean intensity score |
|
|
Pregnant |
16-20 |
17.87 a ± 1.46 |
|
Non pregnant |
6-15 |
10.24 b ± 1.57 |
a, b- Means with different superscript vary significantly (P< 0.05)
The conception rate in the present study with Ovsynch protocol was 58 percent (Table 3) and is in close agreement to the results obtained by Holtz et al. (2008). The conception rate obtained in group II (Progestagen-TRIU-C®) was also 50 percent with four out of eight goats becoming pregnant (Table 3). Motlomelo et al. (2002) reported the conception rate of 46.7 percent using the CIDR regimen of synchronization in Boer goat. Holtz et al. (2008) following a synchronization protocol with FGA (45mg) sponge regimen obtained a conception rate of 46 percent. Thus, the results obtained in the present study are in agreement with the results from the above authors. The conception rates obtained in both the protocols were similar but the conception rate of the synchronized group of animals was higher than that of the control group.
CONCLUSION
Observations from this study suggest that the progestagen (TRIU-C) is more effective than the standard Ovsynch and prostaglandin regimen for the oestrus induction in the goats of tropical region as there was 100 percent oestrous response in progestagen (TRIU-C) group. The oestrus response in group II (Progestagen-TRIU-C®) was 100 percent. There was difference in the time taken for the onset of oestrus between the two groups. The conception rates obtained in both the protocols were similar, however; the conception rate of the synchronized group of animals was higher than that of the control group. Therefore, from the present observations progestagen (TRIU-C®) protocol may be recommended for synchronization of Malabari crossbred goats of tropical region. Moreover, this study suggests that the oestrus intensity has a correlation to the conception rate in goats and this warranties further study.
Funding information
The project was funded by the Post Graduate Student Funding for Sneha Surendra Panicker from Kerala Agricultural University, Kerala.
REFERENCES






