Journal of Animal Health and Production
Research Article
Molecular Identification of Sarcocystis spp. in Meat and Meat Products in Qena, Upper Egypt
Mohamed Abdelfattah Maky1, Eman Sayed Mohammed2*
1Department of Food Hygiene and Control, Faculty of Veterinary Medicine, South Valley University, Qena 83522, Egypt; 2Department of Parasitology, Faculty of Veterinary Medicine, South Valley University, Qena 83522, Egypt.
Abstract | Sarcocystis is a zoonotic protozoan parasite that can be transmitted through the ingestion of undercooked/raw meat contaminated with mature cysts containing bradyzoites. The present study was conducted to investigate the prevalence of sarcocystosis in imported and processed meat products for first time in Qena province, Egypt. A total of 140 specimens of Brazilian imported frozen beef, beef burger, sausage, luncheon, minced meat, kofta (meat balls) and pastrami (20 for each) were collected from several markets in Qena. Specimens were examined macroscopically, by naked eyes, and microscopically, via peptic digestion technique, for detecting Sarcocystis. The identification of Sarcocystis spp. was done by the use of PCR targeting the 18S rRNA gene as well as sequencing of the obtained nucleotides. Our result showed that 58.57% of specimens had Sarcocystis by pepsin digestion technique, while no macroscopic cysts could be detected. The infection rate was 90.0% in imported meat, 75.0% in minced meat, 65.0% in beef burger, 60.0% in luncheon, 55.0% in kofta, 45.0% in sausage, and 20.0% in pastrami. The recovered Sarcocystis species were identified as S. cruzi, S. hominis, S. hirsuta and S. fusiformis. The current work revealed that the high prevalence of microsarcocystosis in imported and processed meat products reflects the potential role of dogs, definitive hosts, rather than cats in the transmission of Sarcocystis spp. in Qena. Interestingly, S. hominis has a zoonotic importance and could be identified in pastrami. Moreover, it is highly recommended that hygienic measures should be applied during meat processing and the consumption of undercooked meat products should be avoided.
Keywords | Egypt, prevalence, meat samples, PCR, Sarcocystis
Received | December 02, 2020; Accepted | December 17, 2020; Published | January 20, 2021
*Correspondence | Eman Sayed Mohammed, Department of Parasitology, Faculty of Veterinary Medicine, South Valley University, Qena 83522, Egypt; Email: emy.hosam@yahoo.com
Citation | Maky MA, Mohammed ES (2021). Molecular identification of sarcocystis spp. In meat and meat products in Qena, Upper Egypt. J. Anim. Health Prod. 9(1): 88-93.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.1.88.93
ISSN | 2308-2801
Copyright © 2021 Maky and Mohammed. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Sarcocystis spp. are cyst-forming zoonotic protozoan parasites of global distribution that poses a threat to the safety of meat/meat products. They are transmitted to humans through consumption of raw or undercooked meat-containing Sarcocystis. Sarcocystis is an obligatory protozoan parasite requiring two hosts, carnivorous and humans as definitive hosts and herbivorous as intermediate hosts, to complete the life cycle. Each host may harbor more than one species of Sarcocystis (Dubey and Lindsay, 2006).
Cattle might be infected with various Sarcocystis species, S. cruzi, S. hirsuta and S. hominis with canids, felids and humans are final hosts, respectively (Tenter 1995; Fayer et al., 2015). Sarcocystis hominis is a zoonotic parasite that infect humans during consumption of raw meat or their products and is the main cause of intestinal sarcocystosis worldwide (Juyal and Bhatia, 1989). Symptoms of human sarcocystosis are abdominal discomfort, nausea, stomach ache and diarrhea (Singla and Juyal, 2014), while the infection of livestock with Sarcocystis spp. may result in weight loss, anemia, abortion and death (in heavy infection) (Fayer, 2014).
The fast food consumption is common in the diets of Egyptian populations, particularly for who spend long times outdoor. Hamburger and sausage are among the most delightful fast foods in various countries. Egyptian beef burger often comprises of minced meat (cattle, sheep, goats, and camels), and other ingredients. Being another popular and cheap fast foods are luncheon and pastrami. Luncheon is precooked or cured meat that sliced and served cold/hot. While, pastrami is frequently prepared from beef meat and may be from sheep or chicken meat. In Egypt, imported meat has been widely consumed because it is cheap. Hence, the careful inspection of meat is mandatory, while the conclusive diagnosis can be achieved by molecular biological tools. Recently, partial 18S rRNA sequencing has been considered as an excellent molecular target gene for characterization of Sarcocystis species (Yang and Zuo, 2000, Pritt et al., 2008).
Based on the scarce data about the prevalence of Sarcocystis in imported and processed meat products in Egypt, particularly the southern districts, the current study was planned to determine the prevalence of sarcocystosis with a special consideration to the identification of the recovered Sarcocystis spp. in raw imported beef meat and processed meat products.
Materials and methods
Samples collection
A total 140 samples (20 samples each) including imported Brazilian frozen meat; sausages, beef burger, Kofta, luncheon, minced meat and pastrami were collected randomly from several markets in Qena province, Upper Egypt. Specimens were examined macroscopically by naked eye and using hand lenses for the presence of macrocysts.
Microscopic examination
For the detection of microcysts, pepsin digestion method has been used. Approximately 2 g of each sample was digested overnight at 40°C in 20 ml of digestion solution containing 3.5 g pepsin, 5 ml HCl, in 500 ml distilled water. The digested material was filtered through a smooth mesh filter and the filtrate was settled for 40 minutes. The sediment was Giemsa-stained and observed microscopically at 400x and 1000x magnifications for the detection of Sarcocystis bradyzoite (Latif et al., 1999; Florencia and Mary, 2000; Saeid et al., 2009).
Molecular identification
DNA extraction: Representative 7 samples were selected, one from each microscopically positive category for molecular identification. DNA extraction was achieved by the use of the QIAamp DNA Mini kit (Qiagen, Germany, GmbH). In brief, 25 mg of the sample was mixed with 20 µl of proteinase K and 180 µl of ATL buffer at 56OC and kept overnight. After incubation, 200 µl of AL buffer was combined with the lysate, incubated for 10 minutes at 72OC, and then 200 µl of 100% ethanol was added to the lysate. The lysate was conveyed to silica column then centrifuged. Washing and centrifugation of the samples were conducted based on the producer’s guidelines. The elution of nucleic acid was done with 100 µl of the supplied elution buffer. The extracted DNA was stored at -20 OC until further processing.
PCR amplification
Oligonucleotide primer used to detect of 18S rRNA gene was supplied from Metabion (Germany) and their sequences were illustrated by Dalimi et al. (2008) Sar-F 5’GCACTTGATGAATTCTGGCA3’ and Sar-R 5’CACCACCCATAGAATCAAG3’. In a 25 µl reaction volume enclosing 12.5 µl of Emerald Amp Max PCR Master Mix (Takara, Japan), 1 µl of each primer of 20 pmol, 4.5 µl of water, and 6 µl of DNA. The PCR was conducted in an Applied Biosystem 2720 thermal cycler program as follows: denaturation at 94ºC for 5 min followed by 35 cycles of 94ºC for 30 sec, annealing temperature was 55 ºC for 40 sec and extension at 72ºC for 45 sec and final extension step at 72ºC for 10 min.
Visualization of the PCR Products
PCR amplicons were detached by electrophoresis on 1.5% agarose gel (Applichem, Germany, GmbH) in 1x TBE buffer. About 5 µl of the PCR products was injected in the gel. Gene marker 100 bp ladder (Fermentas, Germany) was utilized to define the amplicons size. A gel documentation system (Alpha Innotech, Biometra) was used for recoding the result.
Sequencing and genotyping of isolates
PCR products were purified using QIAquick PCR Product extraction kit. (Qiagen, Valencia). Bigdye Terminator V3.1 cycle sequencing kit (Perkin-Elmer) was utilized for the sequence reaction and the purification process was conducted utilizing Centrisep spin column. DNA sequences were obtained by Applied Biosystems3130 genetic analyzer (HITACHI, Japan), were analyzed by a BLAST® analysis performed for sequence identity (Altschul et al., 1990). The phylogenetic tree was constructed by the MegAlign module of Laser gene DNA Star version 12.1 (Thompson et al., 1994). The analyzes were carried out utilizing maximum likelihood, neighbour joining and maximum parsimony in MEGA6 (Tamura et al., 2013).
Statistical analysis
The statistical analysis was performed using SPSS 16.0. The p≤0.05 level was considered as significant.
Table 1: The prevalence of Sarcocystis spp. in meat samples.
| Samples | Number | Positive macroscopic samples | Positive microscopic samples | ||
| No. | % | No. | % | ||
| Imported meat | 20 | 0 | 0 | 18 | 90 |
| Minced meat | 20 | 0 | 0 | 15 | 75 |
| Burger | 20 | 0 | 0 | 13 | 65 |
| Luncheon | 20 | 0 | 0 | 12 | 60 |
| Kofta | 20 | 0 | 0 | 11 | 55 |
| Sausage | 20 | 0 | 0 | 9 | 45 |
| Pastrami | 20 | 0 | 0 | 4 | 20 |
| Total | 140 | 0 | 0 | 82 | 58.57 |
Results
The prevalence of sarcocystosis by microscopic examination was 58.57% (82/140). However, macroscopic Sarcocystis could not be detected (Table 1). Moreover, the infection rate of Sarcocystis in imported beef meat was 90.0%, while prevalence of sarcocystosis in processed beef meat products were 75.0%, 65.0%, 60.0% 55.0% and 45.0% in minced meat, beef burger, luncheon, kofta, sausage and pastrami, respectively.
Meanwhile, the highest prevalence of Sarcocystis spp. in imported meat was 90.0%, while, the lowest infection rate was recorded in pastrami (20.0%). The statistical analysis showed that the infection rate of Sarcocystis in imported meat was significantly higher than that of sausage (p=0.037) and pastrami (p=0.000). The prevalence of Sarcocystis in minced meat was significantly higher than in pastrami (p=0.004) and that in burger was significantly higher than pastrami (p=0.037).
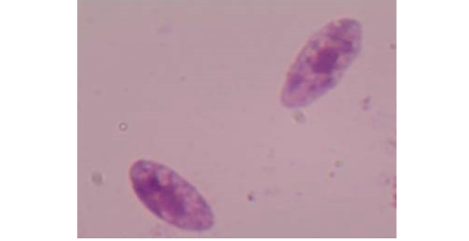
The morphometric characters of the recovered Sarcocystis spp. was clear after the digestion of the peri-cyst tissues and the true cysts were visualized under a light microscopy at 400x magnification (Figure 1). Furthermore, the PCR outcomes showed that all specimens (n=7) had a positive diagnostic band at 600 bp indicating the existence of Sarcocystis spp. (Figure 2).
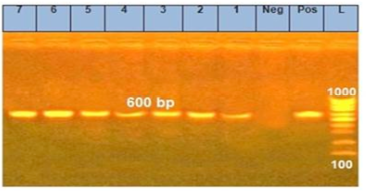
Figure 2: PCR products of 18Sr RNA gene for Sarcocystis species in examined meat samples L: ladder, Neg: negative control, pos: postive control,1: Imported meat, 2: Pastrami, 3: Launcheon, 4: Minced meat, 5: Sausage, 6: Beef burger, 7: Kofta
Moreover, genotyping and sequencing of seven positive isolates were achieved by matching the obtained sequences with the Sarcocystis DNA sequences accessible in GenBank. As a result of the sequence investigation of the 18S rRNA, 4 Sarcocystis species were identified with 100% identity. The identified species were S. cruzi, S. hominis, S. fusiformis and S. hirsuta (Figure 3).
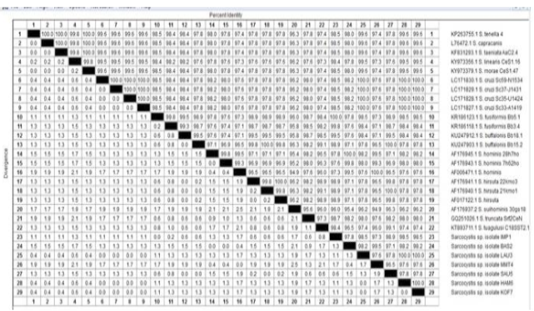
Figure 3: The percentage of identity of Sarcocystis species isolated from beef burger (HAM6), luncheon (LAU3), minced meat (MMT4) and pastrami (BAS2), imported frozen beef (IMP1) and sausage (SAU5)
In addition, S. cruzi was identified in launcheon, kofta and beef burger, S. hominis was detected in pastrami and minced meat, while S. fusiformis was recognized (although no macroscopic cysts was found) in imported frozen beef and S. hirsuta in the sausage (Table 2). The phylogenetic tree of 18S rRNA gene sequences was constructed with the revealed sequences made numerous clusters with reference isolates of global Sarcocystis species (Figure 4).
Table 2: Identified species of Sarcocystis spp. in meat specimens.
| Samples |
Sarcocystis species detected |
Identity % |
| Imported meat | S.fusiformis | 100 |
| Minced meat | S.hominis | 100 |
| Burger | S.cruzi | 100 |
| Luncheon | S.hominis | 100 |
| Kofta | S.cruzi | 100 |
| Sausage | S.hirsuta | 100 |
| Pastrami | S.hominis | 100 |
Discussion
In the present study, macroscopic and microscopic examination as well as the molecular identification were used to investigate the prevalence of Sarcocystis infection in imported Brazilian frozen beef and processed beef meat products. It has been found that an overall prevalence of sarcocystosis was 58.57%. The infection rate of sarcocystosis in Brazilian frozen imported beef was 90.0% that was fully in line with the findings obtained by Mowafy, (2003) in Egypt who stated that the prevalence of Sarcocystis in beef was 100%. However, the achieved findings disagreed with Hussein et al. (2017) who revealed that the prevalence of Sarcocystis in imported frozen Brazilian beef was 6.0% in Egypt. Recently, rejection of beef from Brazil due to Sarcocystis spp. infection was reported by the rapid alert system for food and feed (RASFF) adopted by the European society (Moré et al., 2011). In Iran, a higher infection rate (80.0%) was recorded in processed meat (Dehkordi et al., 2017).
Moreover, previous literature concerned with the prevalence of Sarcocystis spp., in processed meat product worldwide. In this study, minced meat showed a high prevalence of sarcocystosis (75.0%) which was nearly close to the study done by Sancak et al. (1995) (78.5%) in Turkey and it was lower than those recorded by Davoudi et al. (2017) (92.8%) in Ghazvin province, Iran. The higher infection rate of Sarcocystis spp. in minced meat was obtained by Kamber et al. (2018) (28.0%) in Turkey and Meistro et al. (2015) (64.0%) in Italy.
In the present work, the prevalence of Sarcocystis in beef burger was 65.0%. It was harmonious with those of reports by Hajimohammadi et al. (2014) (67.8%) in Iran and it was higher than other studies done in different provinces in Iran; Jahed Khaniki and Kia (2006) (6.25%), Hosseini et al. (2007) (47.9%) and Nematollahia et al. (2013) (56.25%).
Currently, the prevalence of Sarcocystis in kofta was 55.0% which was lower than that reported by Kamber et al. (2018) in Turkey who found the prevalence of sarcocystosis in kofta was 65.0%. It has been reported that the prevalence of Sarcocystis spp. in sausage was 45.0% which nearly close to that given by Maskar et al. (1971) in Turkey who recorded the prevalence of 50.0% and higher than that revealed by Rahdar and Salehi (2011) (8.0%) in Iran and Kamber et al. (2018) 2% in Turkey. Oppositely, Sancak et al. (1995) recorded a higher infection rate in sausage (73.0%).
Few reports demonstrated the prevalence of Sarcocystis spp. in pastrami such as Maskar et al. (1971) in Turkey who found the prevalence of sarcocystosis in pastrami was 88.0%. The ill-developed regulatory policies might be the cause of higher infection rates of sarcocystosis in the past (Kamber et al., 2018). The lower prevalence in our study (20.0%) might be attributed to the good quality of raw meat and associated ingredients. Moreover, the processing of pastrami including drying and salting may prevent the development of the cysts.
It revealed that the current imported meat had the highest infection rate with Sarcocystis spp. compared to the processed meat. This might be referred to the impact of processing technology of meat products and the differences in the composition of products, as the imported meat were produced from crude meat while other products were manufactured from large quantities of fat (Kamber et al., 2018).
Felids are known to be the definitive host of macroscopic Sarcocystis, while dogs are the definitive host of microscopic cyst (Dubey et al., 1989). In the current work, macroscopic cysts were lacked. The microscopic cysts were predominant, which were likely due to the wide spread contact between dogs and cattle; dogs are commonly found on pastures even in/around abattoirs, whereas cats could be seen in urban districts. On the other hand, the feeding habits of canids on raw offal in abattoirs permit the ingestion of cysts and keep the life cycle dynamic and subsequently advance the spread of infection with sporocysts, the main source of infection to cattle. In such way, the infection of intermediate hosts play an imperative role in stimulating a high prevalence of Sarcocystis. Moreover, humans are regarded as intermediate hosts and are at risk for the consumption of undercooked flesh from diseased animals. This will lead to the intestinal sarcocystosis that has a serious impact on the human health (Bunyaratvej et al., 2007).
Four species of Sarcocystis were identified; the predominant one was S. cruzi that was recognized in kofta, beef burger and launcheon, followed by S. hominis in pastrami and minced meat, S fusiformis in imported frozen beef and S. hirsuta in the sausage. Furthermore, the findings were consistent with those reported by Hussein et al. (2017) who detected S. fusiformis in imported Brazilian beef and with Hooshyar et al. (2017) and Hajimohammadi et al. (2014) who revealed S.cruzi in burger. Meistro at al. (2015) detected S. hominis in minced meat. The outcomes of the current study are S. cruzi is a serious effect on the animals with no risk for humans. Sarcocystis hominis is a zoonotic species and concern in relation to the public health, therefore, its diagnosis is crucial in meat presented for human diet, as well as in live animals and even handling with minced meat.
To the best of our knowledge, this study is the first to report S.cruzi in luncheon, S. hominis in pastrami and S. hirsuta in the sausage.
Conclusion
The current study supported the importance of careful inspection of meat and related meat product using microscopic and molecular tools. Consumers have to be aware to the hazards of sarcocystosis especially for those who consume fast food as a main diet in the daily life. Thus, to avoid human sarcocystosis, the meat should be frozen at -20ºC for one day or at -4ºC for 2 days. Meanwhile, meat and their products must be sufficiently cooked at a temperature of 70ºC prior to consumption. Finally, stray dogs should be kept away from abattoirs as they constitute the principal host for spreading microsarcocystosis.
acknowledgegments
We thank Faculty of Veterinary Medicine, South Valley University, Qena, Egypt.
Conflict of interest
Authors declares that there is no conflict of interest
authors contribution
Mohamed Abdelfattah Maky and Eman Sayed Mohammed organised the research, conducted the experiments and created the manuscript.
References