Advances in Animal and Veterinary Sciences
Research Article
Bacteriological and Histopathological Studies on Vibrio Species Isolated from Naturally Infected Freshwater Fish in Delta Region, Egypt
El-Sharaby S. M. A1, Abd-Elgaber M2, Tarabees R3*, Khalil R. H4, Ali M. N.1, El-ballal S.5
1Department of Fish Diseases, Animal Health Research Institute, Dokki, Giza, Egypt; 2Department of Pathology, Faculty of Veterinary Medicine, Menoufia University, Egypt; 3Department of Bacteriology, Mycology, and Immunology, Faculty of Veterinary Medicine, University of Sadat City, Egypt; 4Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, Alexandria University, Egypt. 5Department of Pathology, Faculty of Veterinary Medicine, University of Sadat City, Egypt.
Abstract | The study was aimed to elucidate the most predominant vibrio serovars isolated from naturally infected freshwater fish in the delta region, Egypt. The contamination of water with Cupper and Iron was evaluated. Fishes (n=200) were examined clinically and upon post-mortem. Bacteriological identification was confirmed based on the cultural characteristics and biochemical activities of isolates. A total of 5 isolates of Vibrio harveyi and Vibrio alginolyticus were further examined for the presence of hemolysins (vhh) and collagenase encoding genes using PCR, respectively. The data revealed that Vibrio species were recovered from 39% (78/200) of the examined fishes, and the highest prevalence rate was observed for Angillaangilla (55%) while the lowest was observed for Chrysichthys auratus and Oreochromsniloticus (35%). The V. harveyi was the predominant isolate (29.48%, 23/78), followed by V. anguillarum (28.20%, 22/78), V.vulnificus (16.67%, 13/78), V. alginolyticus (15.38%, 12/78), and V. fluvialis (10.25%, 8/78). Among five examined Vibrio harveyi and Vibrio alginolyticusisolate, a total of 4 (80%) were found to harbor hemolysins (vhh) and collagenase, respectively. Areas where vibrio was recovered in a high percentage, water contamination with Cupper and Iron was higher than the permissible limit. Taken together, the obtained information established the emergence of V. harveyi as a widespread Vibrio serovar warrants further studies to elucidate its public health impact.
Keywords | Vibrio, Freshwater fish, Virulence genes, Heavy metals, Egypt
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | September 17, 2017; Accepted | Deccember 11, 2017; Published | December 28, 2017
*Correspondence | Reda Tarabees, Department of Bacteriology, Mycology, and Immunology, Faculty of Veterinary Medicine, University of Sadat City, Egypt; Email: reda.tarabees@vet.usc.edu.eg
Citation | El-Sharaby SMA, Abd-Elgaber M, Tarabees R, Khalil RH, Ali MN, El-Ballal S (2018). Bacteriological and histopathological studies on vibrio species isolated from naturally infected freshwater fish in delta region, egypt. Adv. Anim. Vet. Sci. 6(1): 17-26.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.1.17.26
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 El-Sharaby et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Fish is a food of high dietary importance providing a good source of high-quality protein, vitamins, and minerals (Hajeb and Jinap 2009). Aquaculture is the supplier of one-third of the entire fish supply worldwide (FAO 2003). Deprived ecological conditions are the main stresses adversely affect the fish immune status and make them immuno compromised and become frailer to bacterial infections (Hansen and Olafsen, 1999; Schulze et al., 2006). Bacterial infections of fish specifically infection with Gram-negative bacteria are still the foremost fret for fish industry (Toranzo et al., 2005). Vibrio species are Gram-negative bacteria commonly isolated from different ecosystems as well as aquaculture farms (Shruti and Haldar, 2012). Vibriosis is a disease of marine and aquaculture reared fishes caused mainly by several Vibrio species. The disease is widely accused of high mortality rate amongst fishes in marine and aquaculture system worldwide (Lavilla-Pitogo CR et al., 1998; Shruti and Haldar, 2012). V. harveyi, V. parahaemolyticus, V. alginolyticus, V. anguillarum, and V. vulnificusare the major Vibrio species infecting marine and aquaculture fishes worldwide. Noteworthy mentioned, V. anguillarum and V. vulnificusare the common Vibrio species infecting fishes in aquaculture ecosystem (Austin and Austin, 2012; Shruti and Haldar, 2012).
The severity of any bacterial infection is mediated mainly via an array of virulence encoding genes that facilitate adhesion, colonization and toxin production and hence disease. The pathogenic V. harveyi is known to harbor many virulence encoding genes, including hemolysins (vhh) (Parvathi et al., 2009), and lots of literature of works established the use of vhh genes targeted PCR as a proof of pathogenic V. harveyi (Castroverde et al., 2006; Conejero and Hedreyda, 2004). Collagenase virulence encoding genes have been shown to be produced by various species of bacteria, including Vibrioalginolyticus for more details about the precise role of collagenase in the pathogenesis of bacterial diseases including Vibriosis see (Harrington, 1996).
Aquaculture systems are presented to various toxins that mainly discharged from effluents released from businesses, sewage treatment plants and seepage from urban and farming regions. These toxins cause genuine harm to fishes (Al – Masri et al., 2002; Karbassi et al., 2006). The contamination of water with heavy metals has turned into a matter of tremendous worry during the last two decades, not just as a result of the danger to open water supplies, additionally it significantly affect the aquatic life structures. The waterway frameworks might be too much polluted with heavy metals discharged from residential, mechanical, mining and horticultural effluents (Vander Oost et al., 2003). Contamination of water with heavy metals will likely modify the biochemical and physiological parameters of fish and hence will magnitude the severity of bacterial infection (Vinodhini and Narayanan, 2009).The present study was undertaken to determine the common Vibrio species affect wild and cultured fishes collected from different localities in Menofiya and Kafr El-Sheikh Provinces. In addition, the histopathological lesions of internal organs were recorded. Ultimately, the association between the contamination of the water with heavy metals and the incidence of Vibriosis was elucidated.
Material and Methods
Fish
A total 200 fishes (80 ± 20 gm) of six main species as folow; 80 of Nile tilapia (Oreochromis niloticus), 40 of African Sharp tooth catfish (Clarias gariepinus), 20 of European eel (anguilla) , 20 of Bottle nose (Mormyrus kannume), 20 of Long blade catfish (Chrysichthys auratus) and 20 of Mullet (Mugil cephalus) fwere collected from various territories in Menofia Province (El Khadroyou area, Elatfa locale and Shabra bass district) and from the private fish farms at Kafr El Sheik region, Egypt (Table 1). Fishes were transported alive to the Fish Diseases Department at the Animal Health Research Institute and subjected to clinical examination and bacterial isolation.
Table 1: The fish species, location and number
| Fish species | Location (Province) | No |
| Oreochromis niloticus | Kafr el-sheikh and Menofia |
80 |
| Clarias gariepinus | Kafr el-sheikh and Menofia |
40 |
| Anguilla anguilla | Kafr el-sheikh and Menofia |
20 |
| Mormyrus kannume | Menofia |
20 |
| Mugil cephalus | Kafr el-sheikh and Menofia | 20 |
|
Total |
200 |
Clinical Examination and Postmortem Lesions
Clinical examination of the fishes was carried out according to (Austin and Austin, 2012; Noga, 2010), while the post-mortem examination was carried out according to (Amlacher, 1970).
Bacteriological Identification
Isolation and biochemical characterization of Vibrio
Samples were collected from fish’s gills, kidney, liver, and spleen under full aseptic conditions. The inoculums were further spread over Tryptic soya broth (Difco, Detroit, MI, USA) supplemented with 3 % NaCl and then incubated at 25 °C for 24–48 hrs. For purification purpose, pure colonies were further sub-cultured into Tryptic soya agar supplemented with 3 % NaCl and 5% sheep blood agar media supplemented with 3 % NaCl and incubated for 18-24 hrs at 37 °C. Full identification scheme mainly includes colonial characteristics, Gram stain and biochemical activities of the isolates (Austin and Austin, 2012; Quinn et al. , 2002).
API 20E and API 20 NE
The biochemical characters of the isolates were determined by using the API-20E and API-20NE system. These strips included the following reactions: ONPG 2-nitrophenyl-ßDgalactopyranoside, ADH L-arginine , LDC L-lysine , CIT trisodium citrate , H2S sodium thiosulfate , URE urea, TDA L-tryptophane, IND L-tryptophane , VP sodium pyruvate , GEL Gelatin , GLU D-glucose, MAN D-mannitol, INO inositol, SOR D-sorbitol , RHA L-rhamnose , SAC D-sucrose, MEL D-melibiose , AMY amygdalin, ARA L-arabinose.
VITEK® 2 compact (BioMérieux) biochemical identification
It is an automated microbiological system used for biochemical identification of isolates according to the manufacturer’s procedure (Malainine et al., 2013).
Genotypic Characterization of Some Virulence Encoding Genes in V. Harveyi and V. Alginolyticus
Genotypic characterization of the hemolysins encoding gene (vvh) and collagenase encoding gene in V. harveyi and V. alginolyticus respectively was carried out according to the protocoal previously established (Di Pinto et al., 2005; Maiti et al., 2009). Briefly, Genomic DNA extraction was carried out using 24 hrs cultures by boiling at 100 °C for 10 minutes (De Medici et al., 2003). PCR reactions were conducted in a total volume of 50 μl containing 25 μl Green Master Mix, 10 μl DNA template, 5 μl upstream primers, 5 μl downstream primers (Table 2) and 5 μl nuclease free water. For the amplification of hemolysin encoding gene (vvh) the PCR condition was 94°C/ 5 min; 30 cycles of (95 °C/ 1 min, 50 °C/ 1 min, 72 °C /5 min) followed by 72 °C /7 min. While, in the case of collagenase encoding gene, the PCR condition was 94° C/ 5 min; 35 cycles of (95 °C/ 1 min, 50 °C/ 1 min, 72 °C /10 min) followed by 72 °C /7 min.The products of PCR were separated by electrophoresis on 1.5% agarose gel (AppliChem, Germany) to define the fragment sizes.
Table 2: Nucleotides sequences of vhh and collagenase encoding genes primers sets
| Bacteria | Target gene | Nucleotide sequences | Size (bp) |
| V. harveyi | vhh |
F: CTTCACGCTT GATGGCTACTG R: GTCACCCAAT G CTACGACCT
|
235 |
| V. alginolyticus | collagenase |
F: CGAGTACAGT CACTTGAAAGCC R: CACAACAGAAC TCGCGTTACC |
737 |
Histopathological examination
Samples collected from internal organs (gills, liver, spleen, and kidney) were immediately fixed at 10% neutral buffered formalin solution, dehydrated in ascending grades of ethyl alcohol, cleared in methyl benzoate and embedded in paraffin wax. Paraffin sections of 3-5 microns thickness were obtained and stained with hematoxylin and eosin dyes (Bancroft and Gamble, 2002).
Determination of Heavy Metal Contamination Level (Mg/L)
Water samples were collected from different vicinities in Menofiya Province (ElKhadroyou region, Elatfa region and Shabra bass region) and from a private fish farm in Kafr El-Sheikh province, Egypt. The samples were acidified by nitric acid and chilled on icebox for transport to the laboratory for heavy metals determination according to theAmerican Public Health Association (APHA, 1995).
Results
Clinical and Post-Mortem Examination of Fish
The clinical examination of the infected fishes revealed skin darkness, hemorrhages all over the fish body especially at the base offins, tail, and fins rot, abdominal dropsy, hemorrhagic ulceration and detachment of scales in scaly fishes. While, the post-mortem examination of infected fishes revealed congestion of gills and visceral organs, enlargement of different organs(liver, spleen, and kidney) and some cases showed that paleness of liver (Figure 1, 2 and 3).

Figure 1: (A) Naturally infected Oreochromis niloticus fish showing skin darkness and fin and tail rot, (B): Naturally infected Oreochromis niloticus fish showing congested gills, pale and enlarged liver, (C): Naturally infected Clarias gariepinus fish showing hemorrhagic ulceration as well as fin and tail rot, and (D): Naturally infected Clarias gariepinus fish showing pale and enlarged liver and hemorrhages all over the body.
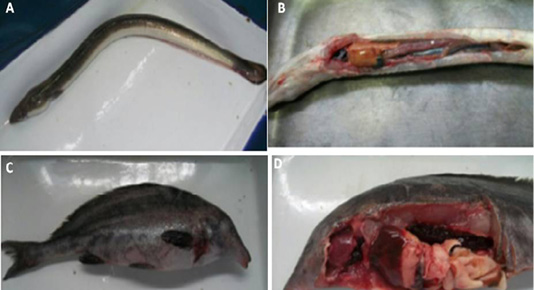
Figure 2: (A) Naturally infected Anguilla anguilla fish showing skin darkness and hemorrhages., (B): Naturally infected Anguilla anguilla fish showing pale and enlarged liver and congested spleen, (C ): Naturally infected Mormyrus kannume fish showing hemorrhages all over the body., and (D): Naturally infected Mormyrus kannume fish showing congestion in different organs (gills, liver and kidney)
Results of Bacteriological Examinations
A- Morphological, culture characters, and Biochemical identification
The bacterial colonies isolated from gills, liver, spleen andkidney ofnaturally infected fishes on Tryptic soya agar supplemented with NaCl (3 %) and on TCBS media at 25ºC for 24-48 hours. V.anguillarum, V. harveyi, V. alginolyticus and V.fluvialis produced yellow colonies on TCBS media. V.vulnificus produced green colonieson TCBS media. All isolated Vibrio spp. were Oxidase positive. V.alginolyticus produced swarming colonies on solid media. Most Vibrio spp. showed curved or bent bacilli when examined by Gram stain. The data presented in Table 3 demonstrated the full identification scheme of isolated Vibrio serotypes.
Table 3: Morphological, culture characteristics and biochemical identification of suspected Vibrio species isolated from naturally infected fishes.
| Criteria | V.anguillarum | V. harveyi | V.vulnificus | V.alginolyticus | V.fluvialis |
| Gram stain | -ve | -ve | -ve | -ve | -ve |
| Growth on TCBS | Yellow colonies | Yellow colonies | Green colonies | Yellow colonies | Yellow colonies |
| Swarming on solid media | -ve | -ve | -ve | +ve | -ve |
| Motility | +ve | +ve | +ve | +ve | +ve |
| Oxidase | +ve | +ve | +ve | +ve | +ve |
| Catalase | +ve | +ve | +ve | +ve | +ve |
| H2S production | -ve | -ve | -ve | +ve | -ve |
|
Urease |
-ve | +ve | -ve | -ve | -ve |
| Citrate | -ve | +ve | +ve | -ve | +ve |
| Indol production | +ve | +ve | -ve | +ve | +ve |
| VP | +ve | -ve | -ve | +ve | -ve |
| Methyl red | (Weak )+ve | +ve | +ve | +ve | +ve |
| Glucose | +ve | +ve | +ve | +ve | +ve |
| Sucrose | +ve | +ve | -ve | +ve | +ve |
| Mannitol | +ve | +ve | -ve | +ve | +ve |
| Lactose | +ve | -ve | +ve | -ve |
-ve |
Table 4: Prevalence of Vibrio species isolated from naturally infected fishes.
| Fish species | No of examined fishes | Positive cases for Vibrio spp. | |
| NO. | % | ||
| Oreochromis niloticus | 80 | 28 | 35 |
| Clarias gariepinus | 40 | 15 | 37.5 |
| Anguilla anguilla | 20 | 11 | 55 |
| Mormyrus kannume | 20 | 8 | 40 |
| Chrysichthys auratus | 20 | 7 | 35 |
| Mugil cephalus | 20 | 9 | 45 |
| Total | 200 | 78 |
39 |
B- Results of Vitek2 system:-
Five isolates were identified using Vitek2 compact. Five isolates were identified as V. anguillarum, V. harveyi, V.vulnificus, V. alginolyticus and V. fluvialis, with a probability of 99%.
Prevalence of Vibrio Species Isolated from Examined Fishes
The prevalence of Vibrio species in the naturally infectedfishes was shown in Table . The data demonstrated that only 39% (78/200) of the examined fishes were infected with Vibrio species, and the highest prevalence rate was observed in case of Oreochromis niloticus (35%) while the lowest prevalence rate was recorded in the case of Chrysichthys auratus (7%). Regarding the incidence rates of different serotypes, the predominated serotypes in the case of Oreochromis niloticus were V.anguillarum (39.3%) V. harveyi (32.1%). While in the case of Clarias gariepinus V.anguillarum (60%) V. harveyi (40%). Conversely, in case of Anguilla anguilla, only V.alginolyticus (63.6%) and V.vulnificus (36.4%) were successfully recovered. While in the case of Mormyrus kannume only V. harveyi (62.5%) and V.vulnificus (37.5%) were successfully confirmed. In the case of Chrysichthys auratus only V. fluvialis (57.1%) and V. harveyi (42.9%) were confirmed. Finally, in the case Mugil cephalus the predominated isolated serotype was V. alginolyticus (55.6%) (Table 4 and 5).
Table 5: Prevalence of Vibrio spp. isolated from naturally infected fishes.
| Fish species | +ve cases | Vibrio spp. | +ve cases | |
| NO. | % | |||
| Oreochromis niloticus | 28 |
V.anguillarum V. harveyi V. vulnificus V. fluvialis |
11 9 6 2 |
39.3 32.1 21.5 7.1 |
| Clarias gariepinus | 15 |
V.anguillarum V. harveyi |
9 6 |
60 40 |
| Anguilla anguilla | 11 |
V.alginolyticus V.vulnificus |
7 4 |
63.6 36.4 |
| Mormyrus kannume | 8 |
V. harveyi V.vulnificus |
5 3 |
62.5 37.5 |
| Chrysichthys auratus | 7 |
V. fluvialis V. harveyi |
4 3 |
57.1 42.9 |
| Mugil cephalus | 9 |
V.anguillarum V. fluvialis V. alginolyticus |
2 2 5 |
22.2 22.2 55.6 |
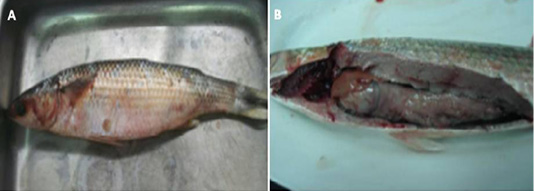
Figure 3: (A) : Naturally infected Mugil cephallus fish showing hemorrhages all over the body, abdominal dropsy and scales detachment , (B): Naturally infected Mugil cephallus fish showing congested gills, pale liver and paleness of intestine.
Table 6: Heavy metal concentrations (mg/L) in water samples from different localities in comparison with the permissible levels.
| Element |
Kafr-Elsheikh (farm) |
Elkhadroyou (region) |
Elatfa (region) |
Shabra- bass (region) | Permissible level |
| Copper | 0.24 | 0.19 | 0. 30 | 0.38 | 0.2 |
| Iron (Fe) | 1.12 | 0.89 | 1.27 | 0.87 | 1.00 |
Genotyping Characterization of Some Vibrio Serotypes Virulence Genes Using Pcr
Genotypic characterization of some virulence determinants in particular hemolysins (vhh) and collagenase virulence encoding genes was carried out using specific primers set for Vibrio harveyi and Vibrio alginolyticus respectively. The data showed that the isolated serotypes exhibited a specific band of 737 bp and 235 bp respectively Figure 4A-B.
Histopathological examination of the examined fishes
The data presented in Figure (5,6 and 7) demonstrated the histopathological changes of the examined internal organs (Liver, spleen, kidney, and gill). Histopathological examination of spleen tissue collected from Clarias gariepinus infected
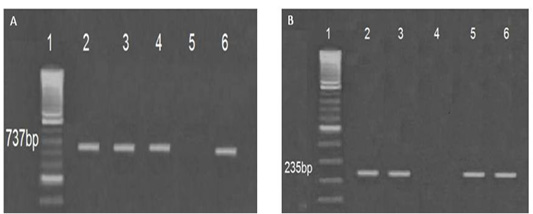
Figure 4: Genotyping characterization of (A) Vibrio alginolyticus lanes (2-6), (737 bp), Lane (1) Ladder (100 bp) and (B) Vibrio harveyi lanes (2-6) (235 bp), Lane (1) Ladder (100 bp) using PCR specific primers targeting and collagenase and hemolysins (vhh) virulence encoding genes respectively.
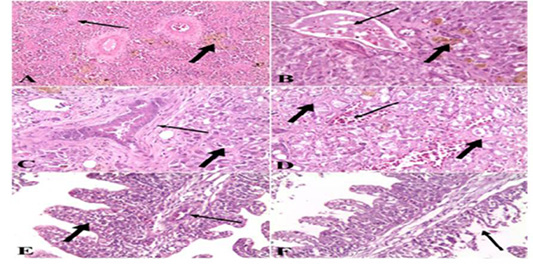
Figure 5: A). Spleen of naturally infected Clarias gariepinus with Vibrio species showed severe fibrosis (thin arrow) and hemosiderin-phages (thick arrow) (H&E x 10); B). Liver of naturally infected Clarias gariepinus with Vibrio species Showed congested portal vein (thin arrow), accumulation of bile pigment (thick arrow) and coagulative necrosis of hepatocytes (H&E x 20); C) Liver of naturally infected Clarias gariepinus with Vibrio species, showed bile duct hyperplasia, increased connective tissue around the bile duct (thin arrow) and coagulative necrosis of hepatocytes (thick arrow) (H&E x 20); D). Liver of naturally infected Clarias gariepinus with Vibrio species showed severe hemorrhages (thin arrow) and proliferation of connective tissue in between hepatocytes (thick arrow) (H&E x 20); E). Gills of naturally infected Clarias gariepinus with Vibrio species showed congested blood vessels (thin arrow), mononuclear cell infiltrations and thickening of secondary lamellae (thick arrow) (H&E x 20); F): Gills of naturally infected Clarias gariepinus with Vibrio species, showed necrosis and desquamation of the secondary lamellae epithelium (arrow) (H&E x 20).
with Vibrio species showed severe fibrosis and proliferation of hemosiderin-phages (Figure 1 A). While in the case of liver samples the data showed congested blood vessels and hepatic sinusoids, severe hemorrhages in the hepatic parenchyma, the hepatocytes showed degenerative changes and coagulative necrosis with an accumulation of bile pigment inside the cells. There is a proliferation of connective tissue fibers between hepatocytes and also in the portal areas hyperplasia of bile duct epithelium (Figure 1 B, C & D). Examination of gill samples collected from Clarias gariepinus infected with Vibrio Species demonstrated severe congestion of blood vessels, mononuclear cell infiltration, thickening and adhesion of secondary lamellae with necrosis and desquamation of epithelium lining some secondary lamellae (Figure 1 E & F). Concerning the histo pathological examination of tissue samples collected from
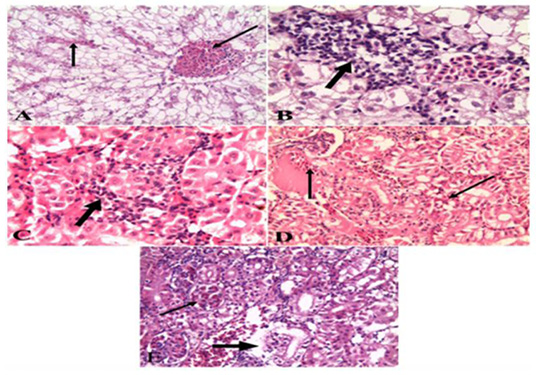
Figure 6: A). Liver of naturally infected Oreochromis niloticus infected with Vibrio Species Showed severely congested blood vessels and hepatic sinusoids (arrow) (H&E x 20); B). Liver of naturally infected Oreochromis niloticus infected with Vibrio Species Showed mononuclear cell infiltrations (arrow) (H&E x 40); C). Kidney of naturally infected Oreochromis niloticus infected with Vibrio Species, showed mononuclear cell infiltrations around renal tubules (arrow) (H&E x 40); D). Kidney of naturally infected Oreochromis niloticus infected with Vibrio Species showed severe hemorrhages (arrow) (H&E x 20); E). Kidney of naturally infected Oreochromis niloticus infected with Vibrio Species, showed congested glomerular capillaries (thin arrow), degeneration and necrosis of renal tubular epithelium (thick arrow) (H&E x 20).
Oreochromis niloticus infected with Vibrio Species, the data showed that liver showed severely congested central veins and hepatic sinusoids. The hepatic parenchyma showed swelling and vacuolation of hepatocytes with focal and diffuse mononuclear cell infiltrations (Figure 2A & B). While examination of the kidneys revealed severe hemorrhages in the interstitial tissue with mononuclear cell infiltrations around renal tubules. The renal tubular epithelium showed vacuolation, necrosis, and desquamation. The glomerular capillaries appeared congested (Figure 2 C, D & E). As for the gills, the data presented showed severely congested blood vessels and mononuclear cell infiltrations. thickening and adhesion of secondary lamellae with vacuolation, necrosis, and desquamation of epithelium lining some secondary lamellae (Figure 3 A & B). Lastly, histopathological examination of spleen tissue samples showed severe hemorrhages with the presence of high number hemosiderin-phages in between necrotic tissue (Figure 3 C & D).
Heavy Metals Concentration in Water Samples
The level of heavy metal pollution in the water samples where the fishes have gathered specifically the levels of Copper and Iron was outlined in the (Table 6). The information displayed demonstrated that water samples gathered from Kafr Elsheih, Elatfa locale, and Shabrarbass area recorded a most noteworthy higher level of copper 0.24, 0.30 and 0.38 mg/ml respectively. Interestingly, the water samples gathered from Kafr Elsheikh and El atfa district recorded the most elevated amount of Iron sullying 1.12 and 1.27 mg/ml in a specific order. Altogether, the water samples gathered from Kafr Elsheikh and El atfa district are considered exceptionally contaminated with higher levels of Cupper and Iron contrasting the other areas.
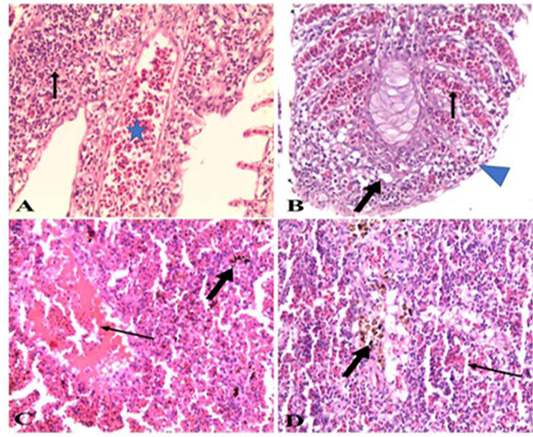
Figure 7: A). Gills of naturally infected Oreochromis niloticus with Vibrio Species showed severely congested blood vessels and mononuclear cell infiltrations (arrow) (H&E x 20); B). Gills of naturally infected Oreochromis niloticus with Vibrio Species, showed severely congested blood vessels (thin arrow), mononuclear cell infiltrations, vacuolation of epithelium (thick arrow) and adhesion between secondary lamellae (arrow head) (H&E x 20); C). Spleen of naturally infected Oreochromis niloticus with Vibrio Species showed severe hemorrhages (thin arrow) and hemosiderin-phages (thick arrow) (H&E x20); D). Spleen of naturally infected Oreochromis niloticus with Vibrio Species showed hemorrhages (thin arrow) and hemosiderin-phages (thick arrow) (H&E x 40).
Discussion
Fisheries in particular aquaculture represent a vital sector in the Egyptian national income. Aquaculture is subject to a variable number of ecological stressors including bacterial infections particularly infection with Vibrio spp. In the present study, a total of 200 freshwater fishes were collected from different localities in Minufyia and Kafr El-sheikh governorates and examined clinically and bacteriologically for the presence of Vibrio species. In addition, the genotypic characterization of Vibrio species was carried out using specific primers was assessed by PCR. Moreover, the association between the infection and the contamination of the water with the heavy metals was assessed. The clinical examination of the fishes showed hemorrhages all over the fish body, at the base of fins, tail, and fins rot, abdominal dropsy, hemorrhagic ulceration, detachment of scales in scaly fishes, redness in the eye and skin darkness. These outcomes are consistent with those previously described (Abd El-Aziz et al., 2013; Ali, 2005; El-Hady et al., 2015; Minami et al., 2016; Younes et al., 2016).The fishes were further examined for postmortem lesions of the visceral lesions. The collected data showed that in some cases, congestion of gills, enlargement ofliver, spleen, and kidney and in some cases paleness of liver was recorded. These results are in agreement with that formerly obtained in many studies (Abd El-Aziz et al., 2013; Ali, 2005; Rameshkumar et al., 2014; Reham, 2009; Stephens et al., 2006; Younes et al., 2016). Although, Vibrio infection in fish can be assumed by clinical signs, however, proper verification of the infection necessitates isolation and identification of the causative agent. Isolation and identification of Vibrio species based on the cultural morphology on selective media and the results of the biochemical tests. The obtained data showed that V. anguillarum, V. harveyi, V. alginolyticusand V.fluvialis produced yellow coloniesand V.vulnificus produced green colonies when cultured on TCBS and V. alginolyticus produced swarming colonies on solid media. The isolated Vibrio spp. were Oxidase positive, and most of the isolates showed curved or bent bacilli on Gram stained film. These results are in agreement with that obtained previously (Chang et al., 2011; Rim et al., 2012; Sabir et al. 2013; Younes et al., 2016).The present study confirmed that the prevalence of Vibrio species amongst naturally infected fishes was 39%. This outcome is lower than that acquired by Adebayo-Tayo and coauthors who demonstrated that Vibrio species were successfully recovered from 44.2% of inspected seafood fish collected from Oron brook (Adebayo-Tayo et al., 2011). Conversely, this result is higher than that obtained by Moustafa and his companions who found that the pervasiveness of Vibrio spp among the tested fishes collected from Qarun Lake at EL-Fayoum region and Suez inlet at Suez governorate was 34% (Moustafa et al., 2010). The discrepancy in results between these studies and the study herein can be attributed to many factors including the water salinity, the number of fishes, species of the fish, season, water quality and area of collection (Lee et al., 2006). In large-scale aquaculture, negative conditions, for example, overcrowding, high temperature, quick development, occasional water restoration rate and despicable evacuation of injured and dead fishes from the cultivating range make great conditions for the rise of bacterial ailments (Almeida et al., 2009). The data obtained herein showed that, the prevalence rate of different Vibrio species isolated from the examined fishes was varied, with V. harveyi is the predominant isolate (29.48%, 23/78), followed by V. anguillarum (28.20%, 22/78), V.vulnificus (16.67%, 13/78), V. alginolyticus (15.38%, 12/78), and V. fluvialis (10.25%, 8/78). This result is not the same as that got previously (Moustafa et al., 2015; Wang et al., 2001), who showed that, V. alginolyticus is the predominant Vibrio serovar recovered from fishes and infection with this serovare usually associated with severe economic losses. V. harveyi is mainly a pathogen of marine aquaculture has been isolated from several diseased marine animals (Hashem and El-barary, 2013; Ransangan et al., 2012). The Nile tilapia Oreochromis niloticus is a fish of both fresh and salt water, and the main pathogenic Vibrio serovars involved in salt water infections are V. anguillarum, V. parahaemolyticus and V. vulnificus. While in freshwater cultures V. mimicus and V. cholerae are the main pathogenic serovars involved (Plumb, 1999; Sakata and Hattori, 1988). Here in, isolation of V. harveyi from fresh water fishes and cultivate farms in Egypt warrants more investigations to clarify its public health impact. Moreovere, additional studies are required to elucidate the pathogenesis of V. harveyi-induced infection in both marine and fresh water fishes.
The virulence of Vibrio pathogen is primarily administered via a variety of virulence encoding genes. Herein, Vibrio alginolyticus and Vibrio harveyi were further tested for the presence of collagnenase and hhv virulence encoding genes respectively using specific primers set. The data showed that, the isolated serotypes exhibited a specific band of 737 bp and 235 bp respectively. This result is consistent with that obtained by others (Di Pinto et al., 2005; Khamesipour et al., 2014; Maiti et al., 2009; Moustafa et al., 2015). PCR has been introduced as an easy, accurate, less expensive, rapid (only takes few hours) tool used for affirmation of pathogenic Vibrio serovar (Bramhachari and Dubey, 2006). The use of PCR as a simple, quick and inexpensivemethod in the diagnosis of Vibrio-related diseases in marine and other large-scale aquaculture systems will help specialists develop accurate preventive and treatment measures to reduce the economic losses associated with these diseases (Shruti and Haldar, 2012).
The histopathological examination of the livers of naturally infected fishes showed congested portal vein, bilepigment, coagulative necrosis of hepatocytes, another showed bile ductin filtration and mononuclear cells infiltrations. Gills showed necrosis, congestion of blood vessels, mononuclear cells infiltrations and thickening of the secondary lamellae. Spleens showed severe fibrosis hemosiderin phages and hemorrhage. While kidneys showed mononuclear cells infiltration around renal tubules, severehemorrhage, congested glomerular capillaries, degeneration, and necrosis of renal tubular epithelium. The obtained data are consistent with the findings obtained by others (Diggles et al., 2000; El-Bassiony, 2001; Korun and Timur, 2008). The presence of histopathological lesions in the internal organs clearly explained the septicemic nature of Vibrio infection. Altogether, the full confirmation of Vibrio infection ought to incorporate, examination of fishes for the visible clinical signs, postmortem examination, isolation of the causative agent using specific media and biochemical tests, and histopathological examination of the internal organs as a proof of septicemic nature of the Vibrio infection.
The contamination of water with heavy metals has been increased markedly during the last decades.Accumulation of heavy metals in marine and freshwater fishes will adversely affect the fishing industry and the consumer’shealth. Therefore, regular monitoring of water for heavy metals contamination is a priority for the food safety regulations (Bosch et al., 2016). In this study, water samples were collected from the areas where fishes collected were examined for the level of heavy metals in particular Copper and Iron. The data presented showed that there is a positive relationship between the incidence of infection with Vibrio and the level of water contamination with Iron and Copper. Contamination of water with heavy metals most likely altered the biochemical and physiological status of the fishes render them more susceptible to infection with bacterial diseases (Vinodhini and Narayanan, 2009). To the best of our knowledge, there are no studies about the precise role of Copper and Iron as heavy metals in the pathogenesis of vibriosis among freshwater fishes. Therefore, the data of this study provides a new finding required further investigation in future studies.This will be a crucial step on the road to reduce stressors on freshwater fishes and thus will legitimately diminish the seriousness of bacterial disease specifically infection with Vibrio spp.
Conclusion
Under the condition of this study, the obtained data revealed that Vibrio is an important bacterial disease affecting freshwater and cultivate farms fishes with wide range host. V. harevyi is the predominantly isolated serovar from freshwater and cultivate farms fishes. Further investigations are required to elucidate its public health impact. Monitoring of the water for the contamination with heavy metals is a crucial step to minimise the severity of bacterial infections, in particular, infection with Vibrio serovars. These findings expand the previous information of Vibrio-induced infection in freshwater ecosystems and present new findings for future investigations.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors would like to thank all the staff members of the Department of Pathology, Faculty of veterinary Medicine, Sadat City University, Egypt and the Department of Fish Diseases, Animal Health Research Institute, Dokki, Giza, Egypt for their valuable support.
Conflict of Interest
The authors would to like to declare there is no conflict of interest
Authors Contribution
El-Sharaby S, Khalil R, Ali M, El Ballal S, design of the experiment, bacteriological and histopathological work, Abd-Elgaber M, and Tarabees R, bacteriological histopathological and molecular work. Tarabees R and Abd-Elgaber M write the manuscript; all the authors approved the final version of the manuscript.
References






