Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (3): 164 – 170Development of Alternative Approaches for In–Process Quality Control of Rabies Vaccine
Manoj Kumar1, Rabindra Prasad Singh1*, Bina Mishra1, Rajendra Singh2, Gundallahallai Bayyappa Manjunatha Reddy2, Arunkumar Patel1, Saravanan Ramakrishnan1, Praveen Kumar Gupta3
- Division of Biological Products, Indian Veterinary Research Institute, Izatnagar–243122
- Division of Pathology, Indian Veterinary Research Institute, Izatnagar–243122
- Division of Veterinary Biotechnology, Indian Veterinary Research Institute, Izatnagar–243122
*Corresponding author: rpsingh@dr.com
ARTICLE CITATION:
Kumar M, Singh RP, Mishra B, Singh R, Reddy GBM, Patel A, Ramakrishnan S, Gupta PK (2014). Development of alternative approaches for in–process quality control of rabies vaccine. Adv. Anim. Vet. Sci. 2 (3): 164 – 170.
Received: 2013–11–24, Revised: 2014–02–13, Accepted: 2014–02–13
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.3.164.170
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
The present investigation aimed on development of alternative approaches for in process quality control of rabies vaccine. Rabies virus (Pasteur Virus–11 strain) was propagated using 0.1 multiplicity of infection and harvested at 48 hrs. The harvested virus was quantified by fluorescent antibody technique (FAT) and mouse inoculation test (MIT). The results suggested that both tests are equally sensitive for virus infectivity assay. Cell culture propagated virus was inactivated by β propiolactone (BPL) and tested for any residual infectivity in bovine hamster kidney –21 (BHK–21) cells using MIT. MIT revealed absence of residual live virus in vaccine sample. Potency of test vaccine was evaluated using mice by National Institute of Health (NIH) test and compared with reference vaccine (Verorab). Potency of test vaccine was adjusted equal to reference vaccine. Findings with NIH test and rapid fluorescent focus inhibition test (RFFIT) indicated correlation of mean antibody titers with survivability of mice following virulent challenge. Therefore, RFFIT can be used as a backup test for potency estimation in conjunction with NIH test. We adopted post bite immunization schedule using rabbit with 2 different doses and assessment of the humoral immune response by RFFIT. The antibody kinetics of these animals indicated that highest antibody titers (≥300IU) were obtained after 4 initial immunizations (0, 3, 7, 14 days). However, the 5th immunization may be beneficial in cases of vaccines having low antigenic value. This is important in developing countries due to poor storage conditions on account of frequent electric failures and inadequate transportation facilities in rural areas, which may result in vaccine with poor antigenic value.
INTRODUCTION
Rabies is one of the oldest, dreadful and highly contagious diseases known to mankind since early civilization. It is prevalent in all parts of world except Australia, New Zealand, Britain, Japan and Scandinavia. Rabies is a severe and fatal viral disease affecting central nervous system of warm–blooded animals, including man (Van Regenmortel et al., 2000). The virus is usually introduced by a bite wound; some time it may penetrate through intact mucous membranes and the digestive tract (Fischman and Ward, 1968), but not through intact skin. Airborne natural infection is also possible in exceptional circumstances, as in caves harboring large numbers of bats carrying the virus (Constantine, 1967).
In Asia, the main route of rabies virus transmission is through rabid dog bites which are responsible for 96–98% of death from rabies in human. The economic burden in Asia has been estimated to be US$ 563 million. It has been estimated that every year 55,000 people die from rabies worldwide, out of this 31,000 (56%) deaths occurs in Asia, mainly (90%) in rural areas (Knobel et al., 2005). It has been estimated that approximately 20,000 persons die from rabies every year in India (Sudarshan et al., 2007). Dog rabies has remained an important cause of rabies in human, especially in the developing countries (Haupt, 1999). In India, rabies is endemic except Lakshdweep, Andaman and Nicobar, Dadra and Nagar Haveli. States like Manipur, Meghalaya, Sikkim, Arunachal Pradesh, Mizoram and Nagaland have reported occasional cases of rabies while substantial deaths have been reported in all other states of India (Sharma, 1990). This could be due to low population density of stray dogs and isolated geographical locations of these places with rest of the country. The control of disease in endemic regions become easier if there is an effective vaccine and efficient diagnosis.
The potency of rabies vaccine is generally determined in vivo by the NIH (National Institute of Health) test (Seligmann, 1973) as recommended by WHO expert committee on rabies (WHO, 1984). This test is based on two vaccinations of mice at 0 and 7th day followed by an intracerebral challenge with the CVS (Challenge Virus Standard) mouse brain strain of fixed rabies virus on day 14th. The European pharmacopoeia suggests a simplified version of the NIH test for animal vaccine (Bijlenga, 1978). In this test mice are challenged by intracerebral injection two weeks after administration of single vaccine dose by intra peritoneal (I/P) route. This technique results in less sensitivity than NIH test (Barth et al., 1988). For Veterinary rabies vaccine potency test, each dilution of antigen contains 10 mice with single immunization (Council of Europe, 2008; Indian pharmacopoeia, 2007; OIE, 2004) while vaccine for human use contains 16 mice (Wilbur and Aubert, 1996) and 18 mice (Farmacopeia Brasileria, 2004) in each dilution with two immunization seven days apart. Sometimes, these laborious in vivo vaccination–challenge procedures in mice are subject to poor reproducibility, may be due to heterogenicity in mice on account of uncontrolled breeding and variations in challenge procedures used (Barth et al., 1988).
For both practical and ethical reasons, replacement of in vivo potency test by more rapid and more reliable in vitro methods for potency estimation is highly desirable (Rooijakkers et al., 1996). Mouse neutralization test (MNT) is considered as a standard test for assay of rabies antibody titers which consist of neutralizing a constant dose of the previously titrated challenge virus with a series of different dilutions of anti–rabies serum, using mice as an indicator system (Koprowski, 1973). Following successful propagation of rabies virus in cell culture, this method has been replaced by in vitro method; with the same principle as in mice. FAT (fluorescent antibody test) is considered to be a gold standard test for the detection and titration of rabies virus in cell culture. Similarly, Rapid Fluorescent Focus Inhibition Test (RFFIT) has been of immense help in detection and titration of rabies virus antibodies. In RFFIT, determination of antibodies is indicated by a reduction in number of fluorescent foci of virus infected cells. RFFIT has been shown to be more sensitive than the MNT in detecting virus neutralizing antibodies in post vaccinated serum (Smith et al., 1973). RFFIT is more reliable and reproducible than virus neutralization test in mice (Fitzgerald, 1979; Louie et al., 1975). Therefore, critical use of FAT and RFFIT for uniform quality control of rabies vaccine will help us in reducing the use of mice for potency assay. Critical use of these in vitro assays will help us to improve quality of vaccine and also in process monitoring of vaccine production. Further, majority of the laboratories in developing countries are forced to handle rabies virus under inadequate bio–safety and bio–security conditions due to high demand of quality vaccine and public pressure. Therefore production of vaccine with minimum efforts due to inbuilt in–process control will reduce the exposure time of workers to such a fatal virus, at the same will help us in economic production of good quality and effective vaccine. Therefore present study was planned to evaluate a combination of in vitro tests for in process quality control and potency test of rabies cell culture vaccine.
MATERIALS AND METHODS
Cell line, Viruses, Antibodies, Vaccine and Conjugate
BHK–21 clone 13 cells between passage numbers 40 to 53 were used in the present study for the propagation of Pasteur Virus (PV–11) strain of rabies virus. Cells were propagated in Glasgow Minimum Essential Medium (Sigma Aldrich) containing 10% Fetal Bovine Serum (GIBCO) and subsequently maintained using 2% Fetal Bovine Serum. Pasteur Virus (PV–11) strain of rabies virus is a fixed virus strain, which is one of the common vaccine strains adapted to grow in BHK–21 cell line. Rabies laboratory of Division of Biological Products, IVRI is maintaining PV–11 strain of rabies virus having titer of 3x106 FFU/mL. This virus was used to produce working stock and stored at –70ºC for further use. Challenge Virus Standard (CVS) strain of rabies virus is also maintained in the same laboratory as an infected brain tissue. Brain tissue at passage second was used in the present study for the potency assay. Verorab (Sanofi Pasteur SA, France) vaccine having ≥2.5 IU/vial was used as reference vaccine and VINRIG (VINS, Bioproducts Ltd. India) anti rabies serum raised in equine having antibody titer ≥300 IU/mL was used as reference serum for antibody assessment in RFFIT. Rabies anti–nucleocapsid antibody conjugated to FITC (VMRD cat#210–28 RAB) and Bio–Rad (cat#357–2114) was used for FAT and RFFIT to detect and quantify virus in BHK–21 cells.
Experimental Animals
About 2–3 weeks old Swiss albino mice of either sex, weighing about 10–15 grams and adult New Zealand white rabbits were procured from Laboratory Animal Research Section, IVRI. These animals were housed, fed and managed under identical conditions. Use of these laboratory animals has the approval of institute animal ethics committee.
Propagation of Virus for Preparation of Working Seed Virus and Experimental Vaccine
Rabies virus (PV–11 strain) from master stock was propagated using 0.1 multiplicity of infection (MOI), by co–cultivation in BHK–21 clone 13 cells. After 48 hrs of infection, virus was harvested and frozen at –20ºC (Anandan, 2006). Then, thawed and centrifuged at 3000 rpm for 10 min. The cell free virus (working seed) was aliquoted and stored at –70ºC. Similarly working seed virus was co–cultivated using 0.1MOI in BHK–21 cell using 5 stack cell factory or roller bottle for production of experimental vaccine. After 48 hrs of infection, virus was harvested as per method explained previously. The cell free virus was aliquoted and stored at –70ºC for quantification of rabies virus using various methods.
Quantification of Rabies Virus by FAT and MIT
Serial 10 fold dilution of virus sample was prepared in eppendorf tube. 30 µL of BHK–21 cells at a cell concentration of 4 x105 cells per mL was added in each eppendorf tube. After gentle vertexing, 10 µL of samples were added to 72 well mini trays cell culture plate (Terasaki plate) in triplicate wells. Then Terasaki plate was incubated at 37ºC for 48hrs under 5% CO2 tension. After the incubation period, medium was aspirated completely and the cell monolayer was washed with phosphate buffer saline (pH–7.2+ 0.2). Cells were fixed using 80% chilled acetone (80/20 in PBS) at –20ºC for 30 minutes. Acetone was aspirated completely and the plate was air dried at 37C for 30 minutes. After complete drying, Rabies anti–nucleocapsid antibody conjugated with FITC was added at the rate of 10 µL per well to cover the monolayer entirely. Then, terasaki plate was incubated at 37ºC for 30 minutes in humid chamber. After aspiration of conjugate, plates were washed with 1x FA rinsing buffer and then soaked in 10 µL of 1x rinsing buffer for 10 minutes. Rinsing buffer was aspirated and 10 µL of mounting fluid (50% Glycerine in 1x FA rinsing buffer, V/V) was added to all wells. Plate was observed under fluorescent microscope and fluorescent foci were counted for the determination of virus titer in terms of FFU/mL of virus sample.
Mouse Inoculation Test (MIT) was performed on 2–3 weeks old Swiss Albino mice, weighing about 10–15 grams. Serially tenfold virus dilution was prepared in phosphate buffer saline and 0.03 mL virus was inoculated intra–cerebrally to 10 mice per dilution (Atanasiu, 1973). All the mice were observed for any clinical signs specific to rabies or death till 14 days. Any death occurring within 5 days was considered as non–specific. Total rabies specific death in each dilution from 5th to 14th day was recorded. Virus titer was calculated using Reed and Muench formula (Reed and Muench, 1938).
Titration of Challenge Virus Standard (CVS)
Rabies CVS strain infected mice brain was used to prepare 20% brain suspension in CVS diluent (2% Horse serum in PBS, pH–7.6) and stored at –80oC. The protocol for CVS titration in mice was similar as used in MIT.
Preparation of Experimental Rabies Vaccine
Cell free virus harvest and stabilizer was added at the ratio of 1:10. Then pH was adjusted to 8.5 with sterile 1N NaOH. Virus harvest was kept in water bath at 37°C and freshly prepared β-propiolactone (BPL) at the final dilution (1:4000) was added with intermittent shaking. The material was kept in water bath for 2 hrs under constant stirring for uniform mixing and kept at 4°C for 24 hrs. Inactivation process was repeated once again in a separate vessel. This was subjected for test of residual infectivity using FAT and MIT. The 5mL material was filled in each vial and freeze dried using an automated lyophilizer.
Potency Test of Rabies Vaccine
NIH test as recommended by WHO expert committee on rabies for the in vivo potency test of rabies vaccine (WHO, 1984) uses 16 mice per dilution with two immunizations 7 days apart. Alternatively Office International des Epizooties (OIE), Indian pharmacopoeia (IP) and European pharmacopoeia (EP), recommend, single immunization with 10 mice per dilution. The protocol we used for the potency test in mice was done with slight modification, as freeze dried test vaccine was reconstituted in 1ml of freshly prepared PBS and reference vaccine (Verorab) was diluted in 1.25 mL of distilled water to make it ≥1 IU/mL. Four fivefold dilutions of test vaccine and reference vaccine (Verorab) were prepared as 1:10, 1:50, 1:250 and 1:1250 dilutions and inoculated by I/P route to 12 mice per group and control group received only PBS. On 14th day blood sample was collected from all immunized mice for antibody assessment. For the same, serum was separated aseptically and inactivated at 56oC for 30 minutes. On the same day mice were challenged with challenge virus standard (CVS). All mice were observed for 14 days after challenge. Potency of test vaccine was calculated in IU/ml in comparison with the reference vaccine.
Rapid Fluorescent Focus Inhibition Test (RFFIT) for Rabies Antibody Assessment
The antibody response was determined by measurement of the neutralizing antibody titer induced after immunization of mice and rabbit using a RFFIT. RFFIT was performed following the protocol with little modifications by Smith et al (Smith et al., 1996). Two different doses of reference vaccine (Verorab) 100 µL (≥0.5IU), 20 µL (≥0.1IU) and test vaccine 500µL (3x107FFU equivalent to inactivated virus), 100µL (6x106FFU equivalent to inactivated virus) was inoculated on 0, 3rd, 7th, 14th, 28th days following a post bite schedule as recommended by WHO and bleeding of all rabbit was done on 0, 14th, 21st, 28th, 35th days from ear vein. Serum was separated under aseptic condition and inactivated in water bath at 56ºC for 30 minutes. Serial 4 fold dilution of serum sample of mice, rabbit and reference serum were prepared in 10% GMEM. Rabies virus, PV strain having titer of 1.22x107 FFU/mL was diluted 1:100 in chilled 10% GMEM and mixed thoroughly. 32 µL of diluted virus was added to all wells containing serially 4–fold diluted serum and virus control well. Then, cell culture plate was incubated at 37ºC for 1 hr for virus neutralization. After the virus neutralization, 32 µL of BHK–21 cells was added in all wells and incubated at 37ºC for 24 hrs in humidified chamber. Cell monolayer was washed with PBS and fixed with 80% chilled acetone (80/20 in PBS) and plate was kept at –20ºC for 30 minutes. Acetone was aspirated completely and the plate was air dried at 37C for 30 minutes. After complete drying, 15 µL of rabies anti–nucleocapsid FITC conjugate (1/20 in PBS) was added to all wells. Then plate was incubated at 37ºC for 30 minutes in humid chamber. Conjugate was aspirated completely and plate was washed with PBS. 30 µL of mounting fluid was added to all wells (50% Glycerine in PBS, V/V) and the plate was observed under fluorescent microscope. Antibody titer in test serum was calculated in comparison with the reference serum in terms of IU/mL.
RESULTS
Propagation of Rabies Virus for Working Seed, Experimental Vaccine and Virus Quantification
Master seed of rabies virus (PV–11 strain) was co–cultivated in BHK–21 clone 13 cells using 0.1 multiplicity of infection (MOI) and harvested at 48 hrs. Degree of fluorescence, in terms of fluorescence forming units (FFU) was observed under a fluorescent microscope. At 10–1and 10–2 virus dilutions almost all the cells were found to emit intense cytoplasmic fluorescence. On increasing virus dilutions fluorescent foci were appreciated distinctly enabling FFU counts. At a virus dilution of 10–4, five infected cells could be detected showing rabies virus specific fluorescence. Titer of virus was found 4×106 FFU/mL using Reed and Muench method. This virus was further propagated with similar protocol for production of experimental vaccine using cell factory and roller bottle.
The pooled cell free virus was subjected to quantification using FAT and MIT. 5 fold dilutions of pooled virus sample harvested at 48 hrs was titrated by indirect FAT using Terasaki plate. Degree of fluorescence, in terms of fluorescence forming unit (FFU) was observed under a fluorescent microscope. At virus dilutions 1:5, 1:25, 1:125, 1: 625 almost all the cells were found to emit intense cytoplasmic fluorescence. On increasing virus dilution fluorescent foci were appreciated distinctly enabling FFU counts. At 1:15625 dilutions 7 infected cells could be detected showing rabies virus specific fluorescence. Same virus sample was titrated 3 times by indirect FAT and the average titer of virus was found 1.22×107 FFU/mL by using Reed and Muench method.
During the present investigation rabies virus (PV–11) was also titrated in vivo in mice. For this 30µl of 10 fold diluted virus (from 10–8 to 10–1) was inoculated intra cerebrally to each mice. These were observed for 14 days for rabies specific symptoms and death. Death after 5 day was only considered as positive and virus titer was expressed in terms of LD50/mL using Reed and Muench method. The average titer of virus was found to be 107.02 LD50/mL.
Potency Assay of Experimental Vaccine
During the present study, virus inactivation was checked by MIT as well as FAT and both revealed no live virus present in test vaccine. Later, potency test of the test vaccine was done using mice and rabbit model. Subsequent upon immunization mice were subjected to RFFIT and challenge test while rabbit samples were tested using RFFIT. Potency of test vaccine was done in mice and compared with reference vaccine (Verorab). The potency of test vaccine was adjusted equal to Verorab vaccine (≥2.5 IU/mL). In order to avoid non-specific death, data was taken as ratio of mice surviving on 5th day of challenge and day at which all control mice died. Apart from this neutralizing antibody were assessed by RFFIT on the day of challenge (14th days post immunization); in order to establish correlation between antibody titer using RFFIT and survivability of mice after challenge. The present study indicated a direct correlation of survival rate with antibody titer in mice as shown in Table 1 and Figure 1a, 1b.
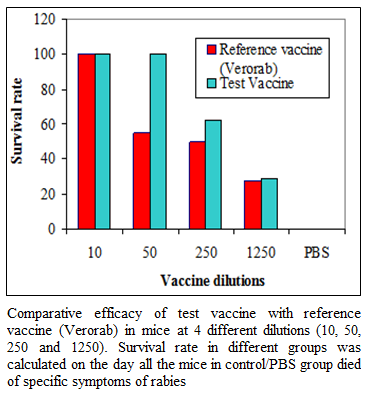
Figure 1a:Comparative efficacy of test vaccine with reference vaccine (Verorab) in mice at 4 different dilutions (10, 50, 250 and 1250). Survival rate in different groups was calculated on the day all the mice in control/PBS group died of specific symptoms of rabies
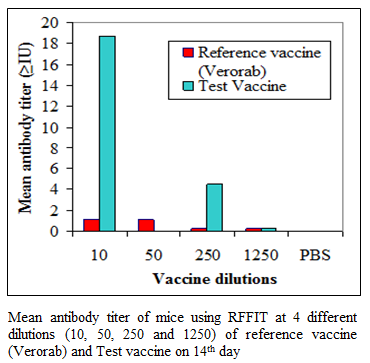
Figure 1b:Mean antibody titer of mice using RFFIT at 4 different dilutions (10, 50, 250 and 1250) of reference vaccine (Verorab) and Test vaccine on 14th day
Table 1 Comparative efficacy of test vaccine with reference vaccine (Verorab) in mice at 4 different dilutions (10, 50, 250 and 1250). Survival rate in different groups was calculated on the day all the mice in control/PBS group died of specific symptoms of rabies. Mean antibody titer of mice as measured by RFFIT was calculated from the sample collected on day 14
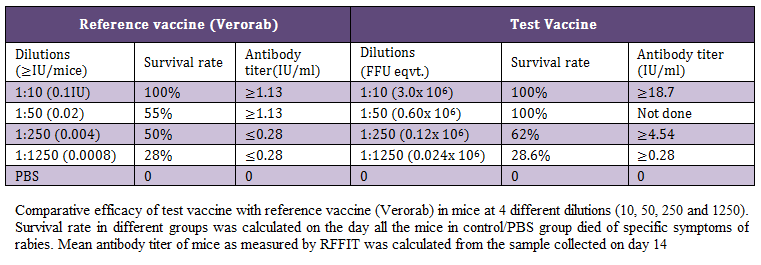
Table 1: Comparative efficacy of test vaccine with reference vaccine (Verorab) in mice at 4 different dilutions (10, 50, 250 and 1250)
Kinetics of Antibody Response in Rabbit
Kinetics of antibody response was investigated at two different doses of reference (Verorab) and test vaccine following post exposure immunization schedule in rabbit using RFFIT. The pattern of antibody kinetics was more or less similar in ≥0.5 IU reference vaccine and 3x107 FFU of test vaccine with slight delayed immune response. At the same titer ≥0.1 IU of reference vaccine (Verorab) produced a similar antibody response as that of 6x106 FFU of test vaccine. The antibody response became optimum in all the groups after 7 days of 5th and final immunization as shown in Table 2.
Statistical Analysis
Test vaccine and reference vaccine data on survival rate were compared after transforming the percent in to degrees using student t–test. It was found that the difference was not significant (p> 0.05).
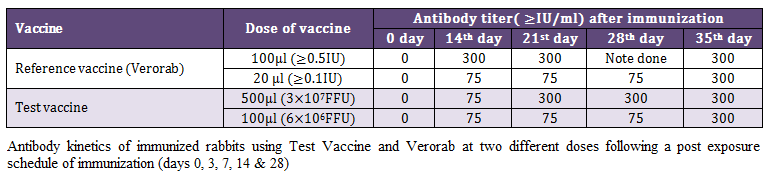
Table 2: Antibody kinetics of immunized rabbits using Test Vaccine and Verorab at two different doses following a post exposure schedule of immunization (days 0, 3, 7, 14 & 28)
DISCUSSION
Sincere efforts to protect the livestock population from rabies have not been given due importance in developing countries. Even though, the disease has been eradicated from many countries with large scale vaccination campaigns and controlled destruction of vectors of the disease (Pastoret et al,, 1992). The majority of the vaccines used in veterinary field at the moment are based upon the use of continuous cell lines such as BHK–21 cells or hamster embryo cell line (NIL2) (Pay et al., 1985; Sureau, 1992). Due to inadequate infrastructure in the developing countries, it is sometimes difficult to get desired antigenic value in the vaccine preparations. A critical observation from virus propagation to production of end product by reassuring certain in–process monitoring system is likely to improve vaccine production and reduce the production cost of the quality vaccine.
Quality of seed virus plays an important role in the vaccine production process. Master seed virus with a titer of 3 x 106 FFU/ mL was taken and subsequently used for preparation of working seed virus with the titer 4 x 106 FFU/mL. This working seed virus was used for the production of an experimental batch of rabies vaccine with a known quantity of virus. For preparation of experimental vaccine, BHK–21 cells were infected using 0.1 MOI of virus and virus harvest was collected at 48 hrs of infection with an idea that this gives a maximum yield of virus (Anandan, 2006). The experimental batch of rabies vaccine was produced in cell factory and roller culture bottle. For comparison, the virus yield in cell factory was 3 times higher than the roller bottle (3 x 107 FFU/mL in cell factory against 107 FFU/mL in roller bottle). It is possible that the cell density and quality of cells is relatively higher in the cell factory as compared to roller bottles. The virus harvest obtained at 48 hrs after inoculation was pooled and subjected for virus quantification and inactivation.
In view of the development of alternative approach for quality control of rabies vaccine, the vaccine virus from experimental batch was quantified using various techniques viz. Fluorescent antibody test (FAT) and Mouse inoculation test (MIT). Comparative efficacy of indirect FAT with MIT indicated that both the tests are equally sensitive. Therefore, these tests can replace each other based on the requirement and level of technical expertise available in the laboratory. The critical analysis of the virus inoculum having a virus titer of 1.22 x 107 FFU/mL by FAT was found to have 107.02 LD50/mL in MIT. This difference could be due to the fact that a part of virus inoculum oozes out during intra cerebral inoculation and also due to measurement errors using insulin syringe. These finding are in accordance with the available literature recommended by WHO and OIE (Chapman et al., 1973; Meslin and Kaplan, 1996, Shankar, 2009). Some of these workers have found that there is a 90–99% correlation between FAT and MIT. Chabara and co–workers (Chhabra et al., 2007) reported that sensitivity of FAT was 100% and it gives 100% coincidence with MIT. False negative FAT results are not common but can occur due to inadequate sampling, faulty equipment, and unsatisfactory conjugate, lack of proper control and lack of experience. Application of FAT as a routine test will reduce use of live animals and time duration (2 days for FAT as against 14 days for MIT).
β–propiolactone (BPL), an alkylating and virus inactivating agent is a most common inactivating agent used for inactivation of rabies virus in the vaccine production process. It has been indicated that in addition to inactivation of virus, BPL also reduces/eliminates the carcinogenic property of BHK–21 cells by strand break and nick of cellular DNA. The damage to the DNA structure by BPL modifies the biological properties of the purified cellular supernatant DNA appraised by its ability to serve as the template in vitro for different polymerases (Morgeaux et al., 1993) and at the same time this does not interfere with the antigenic property of virus for induction of protective immune response. During the present investigation the experimental virus was inactivated using 1:4000 dilution of BPL. This inactivated virus was tested for any residual infectivity in BHK–21 cells using FAT to trace virus infectivity and by mouse inoculation test. Both the test revealed that no residual live virus was present in vaccine sample.
Potency of experimental vaccine was tested using mouse model and the NIH test. For this assay 4 dilutions of reference vaccine (Verorab) and test vaccine were inoculated by I/P route and mouse were challenged with challenge virus strain (CVS) of rabies virus. The potency of 1st dilution of reference vaccine was adjusted to ≥0.1 IU where as that of test vaccine was adjusted equivalent to 3.0 x 106 FFU. The findings indicated that the test vaccine is equally potent as the reference vaccine. It was also observed that 3 x 106 FFU equivalent of inactivated virus may be equivalent to ≥0.1 IU of reference vaccine. Therefore, in order to produce a veterinary vaccine of ≥1 IU, about 3 x 107 FFU infectious units of rabies virus particle may be required. This will fulfill the basic requirement in terms of antigenic value/antigenic mass. Antigenic value would have been more precisely measured by us if, in case the International/National reference standards would have been used in place of reference vaccine as standard. This could not be possible due to non-availability of these standards during the present study. However we need to keep in mind that quantification of rabies antigen is not reliable for estimation of immunogenicity when vaccines derived from different rabies virus strain was compared (Lyng et al., 1992). Antigen concentrations within a given preparation could not correlate with immunogenic potential and not with the ability of a vaccine to stimulate a protective immune response (WHO, 1992).
Antibody assay in immunized mice used for the NIH test seems to be the best possible means to determine the potency of inactivated rabies vaccines (Lazarowicz et al., 1982). The potency may be determined serologically by measuring the neutralizing antibody titers induced after vaccination of mice by using a rapid fluorescent focus inhibition test (RFFIT). Correlation between the challenge test results and the mean titers can be determined by RFFIT. Although this method is faster and less painful for the animals, it is not widely used yet (Kramer et al., 2009). Based on this in present study the humoral immune response was assessed using RFFIT. The antibody response in different group of reference vaccine indicated that ≥0.02 IU of vaccine induces the protective antibody level of ≥1.13 IU/ml while that of about 1.2 x 105 FFU equivalent of test vaccine induces similar protective antibody response. In general the antibody response was higher in test vaccine as compared to respective dilution of reference vaccine. It is worth to mention here that reference vaccine is purified virus: wistar rabies PM/WI–138 1503–3M strain grown in Vero cells while test vaccine is a cell free whole culture of pasture virus–11 strain (PV–11 strain) of rabies virus grown on BHK–21 cells. It is possible that in addition to virus particle, the non-structural component of virus such as soluble glycoprotein (GS) secreted by virus infected cells (Dietzschold et al., 1983), Phosphoprotein (P) or non-structural protein (NS) (Sonoda et al., 1993) and RNA dependant RNA polymerase (L) may also contribute the humoral immune response and also potency of the vaccine.
The major objective of present investigation was to develop an alternative approach for quality control of rabies vaccine which is intended to be used mainly in post bite cases, we adopted similar regime for immunization of rabbit and assessment of the humoral immune response using RFFIT. For this rabbits were immunized on 0, 3, 7, 14 and 28 days at 2 different doses of verorab (≥0.5 IU and ≥0.1 IU/dose) and test vaccine (3 x 107 FFU equivalent and 6 x 106 FFU equivalents). The antibody kinetics of these animals on day 0, 14, 21, 28 and 35 indicated that all the animal show ≥75 IU/ml on 14th days of immunization schedule. A higher early antibody response was observed in rabbit immunized with >0.5 IU on day 14th, which subsequently became equal to test vaccine on day 21st in higher dose of test vaccine. The lower doses (≥0.1 IU/dose) reference vaccine and test vaccine (6 x 106 FFU eqvt.) had an identical antibody response showing protection on day 14 (≥75 IU/ml) which reach the peak (≥300 IU) on day 35 following 5th and final dose of immunization. These finding suggest that although in majority of the cases highest antibody titers are obtained after 4 initial immunizations (0, 3, 7, 14 days). However, the 5th immunization may be beneficial in cases of vaccines with low antigenic value, as is possible in the absence of stringent quality control measure and also poor storage condition in the countries where electric failures are common. This is more important in the rural areas of India which encounter frequent electric failures with inadequate transport conditions and poor awareness about the vaccine quality.
The present investigation reveals that FAT and RFFIT can replace MIT and Mouse virus neutralization test (MNT) respectively during in–process quality control of rabies vaccine. Therefore critical use of FAT and RFFIT for uniform quality control of rabies vaccine will help us in reducing the use of mice for potency assay. Further in order to reduce the number of mice in NIH test/any similar test as recommended by Indian pharmacopoeia and European pharmacopoeia can be used only for the quality assurance of the finished products for release of vaccine batches. Looking at the incidence of vaccine failures in post bite cases of animals, we may focus more on potency of the vaccine with even a slight compromise on purity of antigen. The non-purified vaccine which may have some non–structural proteins of virus origin may induce protective antibody response several fold higher than the affinity purified vaccine. The investigation would have been more interesting and precise with the availability of International reference standards (vaccine/serum). The availability of uniform quality of mice in adequate number may also have improved the authenticity of findings further. Use of different adjutants and their combination may improve the potency of vaccine and may reduce the production cost of vaccine for use in post bite cases of large and small ruminants.
CONCLUSION
The findings of present investigations suggest that FAT for virus quantification and RFFIT for potency test of rabies vaccine is a good option to adapt for in–process quality control of rabies vaccine replacing mouse inoculation test and mouse neutralization test respectively. Quantification of seed virus is crucial for production of good antigenic mass and about 3X107 FFU may be required to produce vaccine of 1 IU. Further, as per the recommended post exposure immunization schedule, 5th inoculation may be important when vaccines with compromised quality /low antigenic value are used in the field. In future, use of mice can be avoided except for final potency testing of rabies vaccine.
ACKNOWLEDGMENTS
The authors thanks the Director, Joint Director (Academic) and Head Division of Biological Products, Indian Veterinary Research Institute (IVRI), Izatnagar, Bareilly, India for providing the facilities and support to carry out this research work. Authors are also thankful to Dr. Sanjay Kumar, Principal Scientist of ARIS cell for statistical analysis of the data generated.
REFERENCES
Anandan P (2006). Growth kinetics of rabies virus in BHK–21 cells. M.V.Sc. Thesis, Deemed University Indian Veterinary Research Institute, Izatnagar.
Atanasiu P (1973). Quantitative assay and potency test of anti–rabies serum and immunoglobulin. In: Laboratory Techniques in rabies. 3rd ed.WHO, Geneva. 314 – 318.
PMid:4219502
Barth R, Diderrich G, Weinmann E (1988). NIH test, a problematic method for testing of inactivated rabies vaccine. Vac. 6: 369 – 377.
http://dx.doi.org/10.1016/0264-410X(88)90185-5
Bijlenga, GA (1978). Potency test which simulates natural exposure for measuring post exposure activity. Dev. Biol. Stand. 40: 203 – 208.
PMid:567153
Chapman WG, Ramshaw IA, Crick J (1973). Inactivated rabies vaccine produced from the Flurry LEP strain of virus grown in BHK–21 suspension cells. App. Microbiol. 26(6): 858 – 862.
PMid:4588193 PMCid:PMC379922
Chhabra M, Mittal V, Jaiswal R, Malik S, Gupta M, Lal S (2007). Development and evaluation of an In vitro isolation of street rabies virus in mouse neuroblastoma cells as compared to conventional tests used for diagnosis of rabies. WHO Collaborating Centre for Rabies Epidemiology NICD, New Delhi – 110 054. 2007
Constantine DG (1967). Rabies virus transmission by air in bat caves. U.S. Public Health Service, Washington, DC.
PMCid:PMC1920085
Council of Europe (2008). Rabies vaccine (Inactivated) for veterinary use (monograph no. 0451). In: The European Pharmacopoeia, 6th ed., Strasbourg, France. 790 – 792.
Dietzschold B, Wiktor TJ, Wunner WH, Varrichio A (1983). Chemical and immunological analysis of the rabies soluble glycoprotein. Virol. 124: 330 – 337.
http://dx.doi.org/10.1016/0042-6822(83)90349-5
Farmacopeia B (2004). Fasiculo 4a Edicao, part II– Vacina Contra Raiva Uso Humano, mon.120. Atheneu Ed. Sao Paulo, SP.
Fischman H, Ward FE (1968). Oral transmission of rabies virus in experimental animals. Am. J. Epidemiol. 88: 132 – 138.
PMid:4873635
Fitzgerald EA (1979). A collaborative study on the testing of rabies immunoglobulin (human) by the mouse neutralization test and the rapid fluorescent focus inhibition test. J. Biol. Stand. 7: 67 – 72.
http://dx.doi.org/10.1016/S0092-1157(79)80038-4
Haupt W (1999). Rabies–risk of exposure and current trends in prevention of human cases. Vaccine 17: 1742 – 1749.
http://dx.doi.org/10.1016/S0264-410X(98)00447-2
Indian pharmacopoeia (2007). Rabies Veterinary Vaccine, inactivated (cell culture), Biological Veterinary Monograph, 2241 – 2242.
Knobel DL, Cleavelant S, Coleman PG, Fevere EM, Meltzer M, Miranda ME (2005). Re–evaluating the burden of rabies in Africa and Asia. Bull WHO. 83(5): 360 – 368.
PMid:15976877 PMCid:PMC2626230
Koprowski H (1973). The mouse inoculation test. In: Meslin FX, Kaplan MH, Koprowski H. (eds). Laboratory Techniques in Rabies. 3rd Eds. WHO, Geneva. 85 – 93.
PMid:4219511
Kramer B, Schildger H, Behrensdorf NHA, Hanschmann KM, Duchow K (2009). The rapid fluorescent focus inhibition test is a suitable method for batch potency testing of inactivated rabies vaccines. Biologicals. 37: 119 – 126.
http://dx.doi.org/10.1016/j.biologicals.2009.01.001
PMid:19181541
Lazarowicz M, Kihm U, Bommeli W, Zutter R (1982). Potency testing of inactivated rabies vaccine in mice, dogs and cats. Comp. immunol. Microbial. Infect. 5 (1–3): 233 – 235.
Louie RE, Dobkin MB, Meyer P, Chin B, Roley RE, Hammar AH, Cabasso J (1975). Measurement of rabies antibody: comparison of the mouse neutralization test with the rapid fluorescent focus inhibition test. J. Biol. Stand. 9: 147 – 156.
Lyng J, Benzton MW, Ferguson M, Fitzgerald EA (1992). Rabies vaccine standardization: international collaborative study for the characterization of the fifth international standard for rabies vaccine. Biologicals. 20: 301 – 313.
http://dx.doi.org/10.1016/S1045-1056(05)80051-X
Meslin FX, Kaplan MM (1996). An overview of laboratory techniques in the diagnosis and prevention of rabies and in rabies research, Chapter 2. In: Laboratory techniques in rabies, 4th ed. Meslin FX, Kaplan MM, Kaprowski H, (eds). WHO, Geneva, 9 – 27.
Morgeaux S, Joffret ML, Leclerc C, Sureau P, Perrin P (1993). Evaluation of the induction of specific cytotoxic T lymphocytes following immunization of F1 hybrid mice with rabies antigens. Res. Virol. 140 –193.
OIE (2004). Rabies, in Manual of standards for diagnostic test and vaccine. Office International des epizooties, Paris, France
Pastoret PP, Brrrrochier B, Blancou J, Artois M, Desmettre P (1992). Development and deliberate release of vaccinia rabies recombinant virus for the oral vaccination of the foxes against rabies. In: Binns MM and Smith GL. (eds). Recombinant poxviruses. CRC Press Inc., London, 163
Pay TWF, Boge A, Menard FJRR, Radlett PJ (1985). Production of rabies vaccine by an industrial scale BHK–21 suspension cell culture process. Dev. Biol. Stand. 60: 171 – 174.
PMid:3899780
Reed LJ, Muench HA (1938). Simple method for estimating fifty percent end points. Am. J. Hyg. 27: 493 – 497.
Rooijakkers E, Groen J, Uittenbogarrd J, Herwijen VJ, Osterhaus A (1996). Development and evaluation of alternative testing methods for the in vivo NIH potency test used for quality control of inactivated rabies vaccines. Dev. Biol. Stand. 86: 137 – 145.
PMid:8785943
Seligmann EBJ (1973). The NIH test for potency. In: Kaplan M, Koprowski H. (eds). Laboratory Techniques in Rabies. WHO, Geneva. 279 – 286.
PMid:4219494
Shankar BP (2009). Advances in diagnosis of rabies. Vet. World. 2(2): 74 – 78.
Sharma RN (1990). Development of inactivated Rabies cell culture vaccine. Ind. J. Virol. 6(1–2): 83 – 86.
Smith JS, Yager PA, Baer GM (1973). A rapid reproducible test for determining rabies neutralizing antibody. Bull. WHO. 5: 902.
Smith JS, Yager PA, Baer GM (1996). A rapid repro¬ducible test for determining rabies neutralizing anti¬body. In: Meslin FX, Kaplan MM, Koprowski H. (eds.). Laboratory Techniques in Rabies. 4th ed. WHO, Geneva, 181 – 192.
PMCid:PMC1295730
Sonoda TY, Fujii H, Mifune K, Ito Y, Minamoto N (1993). Resistance of mice accinated with rabies virus internal structural proteins to lethal infection. Arch.Virol. 132: 51 – 65.
http://dx.doi.org/10.1007/BF01309843
Sudarshan MK, Madhusudana SN, Mahendra BJ, Rao NSN, Narayana A, Rahman AS, Meslin FX, Lobo D, Ravikumar K, Gangaboraih (2007). Assessing burden of human rabies in India: results of a national multi–center epidemiological survey. Int. J. Infect. Dis. 11(1): 29 –35.
http://dx.doi.org/10.1016/j.ijid.2005.10.007
PMid:16678463
Sureau P (1992). Contribution to rabies prevention. Vaccine. 10: 896 – 899.
http://dx.doi.org/10.1016/0264-410X(92)90320-J
Van Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB (2000). Family – Rhabdoviridae. In: Proceedings of the seventh report of the international committee on taxonomy. Classification and nomenclature of viruses. New York Academic press, 563 – 83.
Wilbur LA, Aubert FAM (1996). The NIH test for potency. In: Meslin FX, Kaplan MM, Koprowski H. (eds.). Laboratory techniques in rabies. WHO, Geneva, 360 – 368.
WHO (1984). Report of WHO Consultation on Rabies. WHO Tech. Rep. Ser. N 709.
WHO (1992). Expert committee on rabies, 8th report. WHO Tech. Rep. Ser. 824, 1 – 82.





