Advances in Animal and Veterinary Sciences
Research Article
Role of Gracilaria verrucosa Extract in the Feed as Immunostimulant of White Shrimp (Litopenaeus vannamei) Infected Vibrio harveyi
Sarjito*, Alfabetian Harjuno Condro Haditomo, Kiky Erlinda, Desrina, Slamet Budi Prayitno
Aquaculture Department, Faculty of Fisheries and Marine Science, Universitas Diponegoro, Jl. Prof Sudharto S.H. Tembalang Semarang, 50278, Indonesia.
Abstract | Vibriosis caused by Vibrio harveyi is still a major problem in Pacific white shrimp culture. The development of potential natural compounds to combat bacterial diseases are urgently needed. G. verrucosa, is a marine seaweeds that contains bacteriostatic which is abundance and easily found in all Indonesian marine water. Therefore, G. verrucosa extract could be tested for boosting non-specific immune systems. The research aimed to determine the effect of G. verrucosa extracts in feed on the survival rate, total haemocyte count, and phagocytic activities of white shrimp infected by V. harveyi. A Completely Randomized Design used with five treatments and three replications. The treatments consisted of A (0 g/kg), B (4 g/kg), C (8 g/kg), and D (16 g/kg) of extract/feed respectively. After 14 days fed in various extract concentration, the shrimps were challenge with V. harveyi. The results showed that using G. verrucosa extract the total haemocytes (1.8 X 106cell/mL), phagocytic activity (65%) and survival rate increased up to 79.2%. This results indicating that G. verrucosa extract stimulated antibody responses. The addition of 16 g/kg G verrucosa extract in feed was the best dosage and able to strengthen the white shrimp’s immune response to V. harveyi infection.
Keywords | White shrimp, Gracilaria verrucosa, Immuno-stimulant, Vibrio harveyi, Phagocytic
Received | May 11, 2020; Accepted | September 3, 2020; Published | November 15, 2020
*Correspondence | Sarjito, Aquaculture Department, Faculty of Fisheries and Marine Science, Universitas Diponegoro, Jl. Prof Sudharto S.H. Tembalang Semarang, 50278, Indonesia; Email: sarjito@live.undip.ac.id
Citation | Sarjito, Haditomo AHC, Erlinda K, Desrina, Prayitno SB (2020). Role of Gracilaria verrucosa extract in the Feed as Immunostimulant of White Shrimp (Litopenaeus vannamei) Infected Vibrio harveyi. Adv. Anim. Vet. Sci. 8(12): 1427-1434.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.12.1427.1434
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Sarjito et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Pacific white shrimp (Litopenaeus vannamei) is the most potential commodities of the aquaculture industry that continues to increase. Indonesian shrimp production increased from 24.000 tons in 1980 to 585.000 tons in 2014, which is an approximate increase rate of 65% per year (FAO, 2016; Koto and Fauzi, 2019). In 2017, shrimp commodities accounted for an export of US$ 1.444.333.000, which was 44.15% of the total value of Indonesian fisheries (Fitriani et al., 2019). Despite the potential in shrimp industries, their culture has typical challenges. The most common problem is the presence of a bacterial disease caused by genus Vibrio. V. harveyi that cause mass mortality up to 80% in the larval and juvenile stages before reached three months of intensive shrimp culture.
Thus, increasing shrimp immunity is a major concern for shrimp farmers for disease prevention (Sivagnanavelmurugan et al., 2014; Palanikumar et al., 2020). The farmer tries to find a cheaper and easier local material as a candidate for immunostimulant, such as G. verrucosa, that can be seen as a wild seaweed in Jepara waters.
G. verrucosa has potential bioactive compounds for immunostimulant. Chemical analysis of G. verrucosa shows that seaweed contains high sugar (95.40%) and sulfate (3.4%), which means that the main component of G. verrucosa is polysaccharides (Vienna et al., 2015; Torres et al., 2019). Coarse sulfated polysaccharides extracted from G. verrucosa is potential as shrimp immune-stimulators to increase immunity on the infection (Jasmanindara et al., 2018; Alencar et al., 2019). The sulfate in G. verrucosa bounds to galactose as galactan sulfate, which is proven to be an immune-stimulator in tiger shrimp that is resistant to WSSV (Wongprasert et al., 2014; Cantelli et al., 2019; Rudi et al., 2019). The use of G. verrucosa extract is expected to be an alternative ingredient to prevent V. harveyi infection. It can be used as an alternative control of diseases without endangering the health of consumers and the environment. Arulkumar et al. (2018) and Mubarak et al. (2018) showed that the extracted of G. verrucosa contains steroids, quinones, terpenoids, saponins, flavonoids, and phenolic compounds or tannins that could combat various disease and protect the cells.
Beside immune-stimulant, G verrucosa could also stimulate the growth rate of the shrimp. Jasminandar et al. (2018a) and Ihsan et al. (2019) reported that feed containing 2 g/kg G. verrucosa extract resulted in a higher growth rate of white shrimp. The weight gain of the given shrimp was quite significant compared to control shrimp. A similar result also reported by Sanchez et al. (2018), which showed that the increase in growth rate and specific growth rate of shrimp given by diet extract once a week and once at the beginning of the experiment was significantly higher compared to control shrimp. Therefore, the addition of G. verrucosa extracts in the diet not only able to increase shrimp immunity but also improve the growth performance.
Furthermore, an addition of a small amount of seaweed extract has significantly increasing the growth rate and feed utilization (Giang et al., 2016; Omont et al., 2019). Geographical differences might result in different concentrations of the active ingredient. This research, therefore, to study the immunostimulant potential of G.verrucosa extract.
MATERIALS AND METHODS
This research was conducted from January to April 2019 at the Laboratory of Aquatic Animal Health Management, Brackishwater Aquaculture Development Agency, Jepara. White shrimp (L. vannamei), which were free from vibriosis and WSSV disease, were used in this experiment. The experimental shrimps were obtained from Shrimp Farmer Group ‘Gumuk Mas’, Jepara, Central Java. There were 300 shrimp seeds with the weight ranging from 3 to 5 g, and the length approximately 7±1.2 cm. After selection process, only 96 shrimp juveniles were used and then stocked in twelve aquariums. The stocking density was eight animals per aquarium. G. verrucosa and V. harveyi that were used in the present study were obtained from the Brackishwater Aquaculture Development Agency, Jepara. The diet that used was a commercial shrimp feed.
G. verucossa seaweed was washed with sea water and then rinse in freshwater to remove salt, sand, debris and other undesirable materials. The cleaned seaweed then air dried under direct sunlight for 5 days. After that, the seaweed finely ground and sieved with a fine sieve (60 mesh size). Extraction was done by adding methanolic solvent in a ratio of 1: 3 (w / v), then shaking at 37°C at 135 RPM for 24 hours, settling, and then filtering. The filtrate obtained was evaporated using a vacuum rotary evaporator at 55°C to obtain a paste extract.
The method used in this study was a complete randomized experimental design with four treatments and three replications. The treatment consists of various dosages of G. verrucosa extracts in the diet. The treatments were A (0 g/kg feed), B (4 g/kg feed), C (8 g/kg feed) and D (16 g/kg feed). The experimental shrimps were reared at various treatments for 14 days to stimulate the immune system. The G. verrucosa extract as immune-stimulant in the diet was added by mixing this extract in a 100 mL of distilled water with 4% progol as pellet binder, then sprayed on the surface of commercial feed evenly and dried in room temperature.
The feeding regime was 3.5% body weight of biomass. Feeding was given four times a day at 07.00, 12.00, 17.00, and 21.00 Local Time for 14 days. The challenge test was carried out on the 15th day of culture by injecting 0.1 mL V. harveyi intramuscularly at a dose of 104 CFU/ml. Observed variables consisted of immune responses, clinical symptoms, and survival rates. The immune responses observed were Total Haemocyte Count (THC) and Phagocytic Activity (PA). For THC analysis, 0.1 mL of haemolymph taken at the fifth periopod using a 1 mL syringe filled with 0.3 ml Na-EDTA anticoagulant to prevent blood clots, then homogenized for 5 minutes in a microtube that has been moistened with 10% Na-EDTA Solutions. A total of 0.05 mL haemolymph imposed on haemocytometer and covered with a glass cover that had been cleaned with 95% ethanol for 30 minutes. Then, the cells were counted under a light microscope with 400x magnification. Observations THC challenge tests done once before and four times after the test challenge with V. harveyi infection. Whereas PA was determined by mixing 20 mL haemocytes with 20 mL of bacteria V. harveyi at densities of 107 CFU.mL-1. Incubated the mixture for 30 minutes and then stained. Blood smears were made by dripping 5 μL of the mixture incubated on a glass object and smeared with a glass cover. Streaks as thin as possible, not be interrupted and then dried. Smear is fixed with 95% ethanol for 5 minutes. Staining was performed with 10% Giemsa for 20 minutes and rinsed using distilled water and then dried. Observe under the microscope with 400x magnification, observe at least 100 cells or 10 views. Active and inactive cells and the number of phagocytic bacteria was counted.
THC and PA measurements were conducted at Day 0 and the 14th day prior challenge test and once every three days after the challenge test. Observation of clinical symptoms and survival of shrimp after the challenge test were performed for 14 days. Water dislodging was done every day to maintain water quality.
Intake and preparation of shrimp haemolymph for haemocyte count was performed using the modified procedure of Zahra et al. (2017). The number of haemocytes as determined by referring to the calculation of Blaxhall and Daisley (1973), i.e.:
THC = average Σ counted cells x 250 x Dilution Factor x 1000
Phagocytic activity (PA) was determined using the modified procedure of Sudaryono et al. (2018). A calculation can determine PA values according to Elshopakey et al. (2018), i.e.:
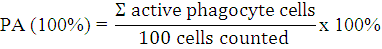
The clinical symptoms were examined by observing morphological behaviour and changes after V. harveyi experimental infection, such as changes in body colour, body condition, appetite, and hepatopancreas conditions by the scoring method.
The shrimp survival rate was observed everyday post-challenge until the end of the experiment. The survival rate (SR) of shrimp was calculated using the formula, according to Ly et al. (2019) as follows:
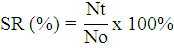
Notes:
SR= Survival Rate (%); Nt= Number of shrimp at the end of the experiment; No= Number of shrimp at the beginning of the experiment.
Statistical analysis
The clinical symptoms, immune response parameters such as Total Haemocyte Count (THC), and Phagocytic Activities (PA) were analysed descriptively. The survival rate was analysed using analysis of variance (ANOVA) at a 95% confidence interval to see the effect of treatments using SPSS 21. When ANOVA demonstrated significantly different, a further Duncan test was performed to determine the best treatment of this experiment.
RESULTS AND DISCUSSION
Clinical symptoms
The clinical symptoms of white shrimp after challenged with V. harveyi were shown in Table 1 and Figure 1. In the 1st day, shrimp exhibited redness on tail nor the tip of the head (Figure 3a) at all treatments and mortality was first recorded in treatment C. In the 2nd day, clinical symptoms such as brownish hepatopancreas, body melanisation, and discoloration start to occur in all procedures, and mortality was counted in treatment A (2 shrimps) and C (1 shrimp). Redness and necrosis on the tail, swimming legs, and body discoloration are presented in Figure 3b, 3c, and 3d, respectively.

Figure 1: Clinical symptoms of infected shrimp: (a) Reddish head and tail in the upper infected shrimp. The lower shrimp is the healthy shrimp, (b) Necrosis tail, (c) Reddish swimming legs, (d) Changes in redness of the body.
Clinical symptoms demonstrated a recovery after the 11th day. Moreover, mortality was also steeply declined (Table 1). However, the sign of redness on the tail, body discoloration, and brownish of hepatopancreas were still detected. No mortalities were found from the 12th day to the end of the experiment (the 14th day).
Total haemocyte count (THC)
Total haemocytes of white shrimp are counted from the day before treatment (Day 0) to the 21st day (the post-challenge of the 6th day) ranged from 0.11 to 1.82 (x106 cells/mL). THC observations during the study presented in Figure 2. THC increased from Day 0 (before treatment) to the 14th day. The increase of THC values on the 14th day found in treatments B and D. The THC were then decreased at treatments A, B, and D for the 1st day after the shrimp had been challenged with V. harveyi on the 16th day. Further, a decrease of THC occurred on the 18th day in treatments A, B, and C, except, in treatment D. While on the 21st day, the THCs in all procedures were decreased except treatment A. However, the total haemocytes count at treatment D was still the highest compared to the other.
Table 1: Clinical Symptoms of Vaname Shrimp after Infected V. harveyi.
| Day | A | B | C | D | ||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| 1 | ++ | ++ | ++ | ++ | ++ | ++ | (-1) | ++ | ++ | ++ | +++ | ++ |
| 2 | (-1) | ++ | (-1) | +++ | ++ | ++ | (-1) | ++ | ++ | ++ | +++ | +++ |
| 3 | (-1) | +++ | ++ | +++ | ++ | ++ | +++ | ++ | ++ | (-1) | (-1) | +++ |
| 4 | +++ | (-1) | (-1) | +++ | (-1) | ++ | (-1) | +++ | (-1) | +++ | +++ | +++ |
| 5 | +++ | +++ | ++ | +++ | ++ | (-1) | +++ | +++ | +++ | +++ | (-1) | (-1) |
| 6 | +++ | (-2) | (-1) | +++ | (-1) | (-1) | +++ | (-2) | +++ | +++ | (-1) | ++ |
| 7 | (-1) | (-1) | (-1) | (-1) | +++ | ++ | ++ | +++ | +++ | +++ | +++ | +++ |
| 8 | +++ | +++ | ++ | +++ | +++ | +++ | ++ | ++ | +++ | +++ | +++ | ++ |
| 9 | +++ | ++ | +++ | (-1) | (-1) | +++ | +++ | ++ | (-1) | +++ | +++ | +++ |
| 10 | (-1) | (-1) | +++ | ++ | +++ | (-1) | +++ | +++ | ++ | +++ | +++ | +++ |
| 11 | +++ | +++ | +++ | +++ | ++ | ++ | ++ | ++ | (-1) | +++ | ++ | ++ |
| 12 | +++ | +++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ | +++ | ++ | ++ |
| 13 | +++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 14 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
Description: +: normal appetite, normal swimming; ++: the reddish on tail nor the tip of the head, brownish hepatopancreas, melanization on the body; +++: changes in body color (body, tail, swimming legs, walking legs, and the tip of the head), necrosis on the tail, brownish hepatopancreas, melanization on the body; (-1),(-2) : each number represents the number of shrimps that have die.
Phagocytic activity (PA)
Phagocytic activities from Day 0 before treatment until the 21st day (after the sixth-day of the bacterial challenge) showed variation ranging from 20% - 68%, as presented in Figure 3. This Figure 3 showed that the PAs slightly increased after the shrimps were fed with G. verrucosa diet, especially in treatment C and D on the 14th day compared to Day 0. The 1st day after being challenged (the 16th day), experimental shrimps exhibited better phagocytic activities, particularly in treatments A, B, and C. While PA in treatment D demonstrated significantly decline. In contrast, on the 18th day, PAs at treatments A, B, and C were declined whilst treatment D was contrary increased. On the 21st day, phagocytic activities at all treatments were decreased, however, treatment D was still at a higher value compared to other treatments.
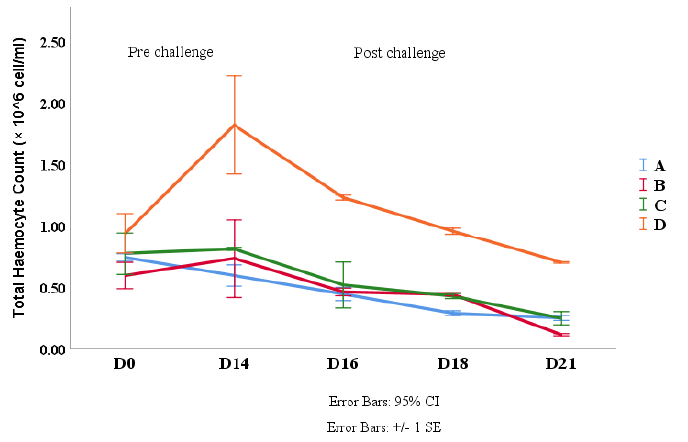
Figure 2: THC Count of Vannamei Shrimp (L. vannamei) at pre and post challenge with V. harveyi. Description: A (0 g/kg feed), B (4 g/kg feed), C (8 g/kg feed) and D (16 g/kg feed).
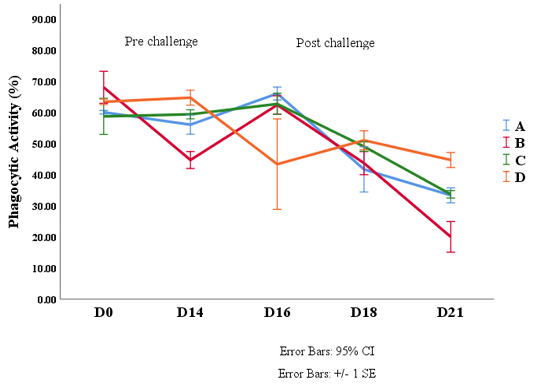
Figure 3: Phagocytic Activity Value of Shrimp Vaname (L. vannamei). Description: A (0 g/kg feed), B (4 g/kg feed ), C (8 g/kg feed) and D (16 g/kg feed).
Survival rate
The number of mortality of white shrimp after challenged with V. harveyi in all treatments was as follows; treatment A, B, C, and D were 13 shrimps, eight shrimps, eight shrimps, and five shrimps, respectively. The survival rate patterns of white shrimp for each treatment for 14 days post-challenge are presented in Figure 4. The mortality began on the 2nd day after the challenge. Deaths continued to increase until the 4th day and reached a peak on the 6th day. After the 7th day, the deaths began to decrease, and no more mortality on the 11th day. The white shrimp feed with G. verrucosa extract demonstrated a better survival rate compared to the control (Figure 4). ANOVA tests showed that the administration of G. verrucosa extracts into the diet for 14 days demonstrated a significant effect (P <0.05) on the survival of vannamei shrimp infected by V. harveyi. Therefore, the research also revealed that the usage of G. verrucosa extract in the diet at a concentration of 16 g/kg was provided the highest survival rate of juvenile white shrimps.
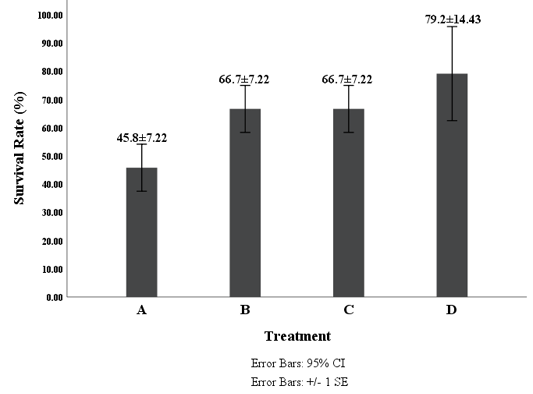
Figure 4: Survival Rate of Shrimp Vaname (L. vannamei) Description: A (0 g/kg feed), B (4 g/kg feed ), C (8 g/kg feed) and D (16 g/kg feed).
Clinical symptoms after 24 hours of V. harveyi infection were a decrease in appetite and the reddish at the tip of the tail and head. A reddish and necrosis on the tail area and carapace, melanisation of the bodies, and browning hepatopancreas happened after 48 hours. The melanisation of the body related to the decrease of total haemocytes will cause damage to the host (Zahra et al., 2017; Qin et al., 2019; Jasmanindarb et al., 2018). Sarjito et al. (2012, 2018) explained that shrimp infected by vibriosis was characterized by the reddish carapace, melanosis of the skin, necrosis on the tail, lesions, and brownish hepatopancreas.
The increase of THC after consuming a medicated feed contained G. verrucosa thought to be influenced by bioactive compound, which could increase shrimp immunity. Jasmanindar et al. (2018b) and Liu et al. (2019) explained that coarse sulphate polysaccharides derived from G. verrucosa reported being immune-stimulators in shrimp. The polysaccharides isolated from seaweed had a molecular pattern that was recognized by shrimp innate immune cells. The different values of THC might be due to varying concentrations of polysaccharides in the diet due to treatments applied.
The present result found that the decrease of THC started on day forth teen after the shrimp infected of V. harveyi. That condition might be influenced by the movement of haemocyte cells from haemolymph to the tissues (Hipolito et al., 2014; Cantelli et al., 2019). It was causing a reduction in haemocyte concentrations in the haemolymph. However, it could also be caused by physiological conditions of shrimps that have infected by bacteria. The number of hemocytes reducing because of the experimental shrimps defended the spread of foreign bodies, such as infiltration of infected tissue and death haemocyte cells due to the mechanism of apoptosis (Zubaidah et al., 2015). Then, the THC pattern was directly proportional to the reduction of PA on the 18th and 21st day. It was due to the existence of phagocyte cells, namely hyaline cells (Sivagnanavelmurugan et al., 2014; Saputra et al., 2019).
The increase of PA in all treatments, except in treatment D after the 1st day challenge, indicated that active phagocytic cells ingested pathogens. As a result, pathogenic bacterial numbers in the body of shrimp reduced. Extract of G. verrucosa in the feed may stimulate phagocytic cells to phagocytic pathogens. Even though, the PA (65%) was higher than Jasmaniar’s Research (2018b) of 44.3 ± 3.5%, but this result in line with Jasmanindara et al. (2018) and Peixoto et al. (2018) that G. verrucosa extract stimulated the immune response in white shrimp. The increase of PA after the 1st day of challenge (the 16th day) reduced the mortality that only one shrimp death. After that, the PAs were declined, and the mortality varied. However, in between the 18th and 21st day was less suspected of committing suicide cell, known as apoptosis after phagocytic resistance to bacterial infection. Chen and He (2019) and Liu et al. (2020) stated that the variations in the number of haemocytes are caused by the responses to infections, species, and environmental stress. The number of haemocytes can decrease drastically during infection by pathogens (Jasmanindarb et al., 2018). The result also indicated that low PAs on the 18th and 21st days correlated with THCs, which also decreases. It can be assumed that low haemocytes in the body of shrimp cause weakening of the non-specific immune system, which makes the shrimp unable to fight against bacterial infections, so resulting in mortality of the shrimp (Yang et al., 2015; Rudi et al., 2019). The high mortality that occurred in each treatment on the 3rd day (the 18th day) until the 6th day of challenged (the 21st day) indicated that the shrimps were in weak condition.
Feeding with the addition of G. verrucosa extract has a significant effect (p <0.05) on the survival rate of shrimp. The result showed that the highest survival rate found in treatment D (79.2%). The highest survival rate in the present study (79.2%) was slightly lower compared to the previous study done by Rudi et al., 2019 that was 80% survival, but this result was higher than the Jasmanindar’s Research (2018a) of 76.7%. Treatment D also resulted in the most top immune response to THC and PA at both 14 days of feeding with diet contained G. verrucosa extract and after the challenge test. It indicated that the addition of G. verrucosa extract improved a degree of resistance of L. vannamei to V. harveyi and at the same time reinforce the immune system. The survival rate of white shrimps was quite high after V. harveyi infection allegedly due to the bioactive compound in G. verrucosa extract. According to Ihsan et al. (2019), the content of polysaccharides in the G. verrucosa extract was thought to stimulate the formation of hemocyte cells to increase shrimp immune response after periodic feeding. The increase of immune response may have an impact on increasing endurance so that shrimp are more resistant to disease. It was confirmed by Trafalgar et al. (2013) and Cantelli et al. (2019) that the increase in endurance seen in the treatment with the addition of extracts thought to be associated with an increased immune response especially from the phagocytic activity which removes pathogens, viruses or invading microbes. Jasmanindara et al. (2018) found that the low survival rate in the treatment without extract seaweed was related to the weaker immune response compared to the treatment with the addition of seaweed extract. This statement was confirmed by Liu et al. (2019) and Maftuch et al. (2012), that such conditions indicated that the magnitude of the immunological response generated by the without seaweed extract was not enough to provide adequate protection against V. harveyi. Peixoto et al. (2018) found that the addition of Gracilaria sp. increases immune response on the European Seabass. This research also found that the survival rate was significantly different between shrimps fed with seaweed extract and shrimps fed without the addition of G. verrucosa extract. Bioactive compound present in G. verrucosa was able to stimulate innate immunity and improve resistance to pathogenic bacteria. This present study revealed that addition of methanolic G. verrusoca extracts of 16g kg-1 could be applied for alternative solution of vibriosis in shrimp culture. It is then strongly recommended that addition of G. verrucosa extract in the diet could be applied to prevent bacterial disease.
CONCLUSIONS AND RECOMMENDATIONS
The addition of G. verrucosa extract into the feed influenced the immune response of white shrimp as indicated by the difference between the total number of hemocytes (THC) and phagocytic activity (PA) during 14 days and after the challenge with V. harveyi. Similarly, the survival rate of shrimps feed with G. verrucosa extract significantly higher compared to control. The infected shrimp that has been fed with G. verrucosa extract seem to be recovered after twelve days post infection. The addition of G. verrucosa extract in the feed at concentration of 16 g/kg showed the best immune response and the highest survival rate of 79.2 %.
ACKNOWLEDGMENT
This research was partly funded by PNBP of Fisheries and Marine Science, Diponegoro University contract number, 11/UN7.5.10/PP/2019. Appreciation was given to the Dean of Fisheries and Marine Science, Universitas Diponegoro, Head Laboratory of Aquatic Animal Health Management of Brackish water Aquaculture, and Development Agency, Jepara, who has provided facilities for this research.
Author’s Contribution
Sarjito designed the experiment, data analysis and preparing the first draft of manuscript. Kiki Erlinda conducting the experiment and collecting data. Alfabetian Herjuno Condro collecting data and supervised the experiment. Desrina and Slamet Budi Prayitno took part in data analysis and improving the manuscript
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






