Advances in Animal and Veterinary Sciences
Research Article
Histomorphological and Histochemical Postnatal Developmental Study of the Pelvic Reproductive Organs in Female Rabbits (Oryctolagus Cuniculus)
F. J. Al-Saffar*, Masarat S. Almayahi
Department of Anatomy, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq.
Abstract | This project was carried out to find out the structural histomorphological changes that take place in the pelvic parts (vagina and vestibule) of the female reproductive system of the rabbits (Oryctolagus Cuniculus) at three different postnatal periods of their life that were kitten, immature and mature does. To conduct such project, twenty four female rabbits were bought (8 for each age) from the local breeders. Rabbits were euthanized, dissected and subsequently vaginae and vestibules were collected and fixed with 10% neutral buffered formalin then subjected to routine processing, such as dehydration, clearing, embedding and block preparation. Finally, sectioning for 6 µm were prepared and stained with hematoxylin-eosin and Masson’s trichrome stains. Gross findings revealed that the vagina and vestibule in the local rabbits were characteristically very long tube-like structures. The vagina entirely was running ventral to the rectum together with vestibule in the pelvic cavity. Microscopic findings revealed prominent structural changes in the vagina of immature does compared to those of kittens and mature does indicated that immature does after the period of weaning face great developmental growth and changes. The microscopic changes in the vestibule were slight alterations and no age related changes were recorded. Statistical analysis revealed that the total thickness of the mucosa plus muscularis of the vaginal wall was decreased significantly in the direction cranio –caudally.In conclusion, the current study recorded differences in both macroscopic and microscopic aspects of the vagina and vestibule of the local rabbits compared to other animal species.
Keywords | Vagina, Vestibule, Rabbit, Does, Kitten
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | September 13, 2018; Accepted | November 01, 2018; Published | November 20, 2018
*Correspondence | FJ Al-Saffar, Department of Anatomy, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq; Email: assaffar1955@yahoo.com
Citation | Al-Saffar FJ, Almayahi MS (2019). Histomorphological and histochemical postnatal developmental study of the pelvic reproductive organs in female rabbits (oryctolagus cuniculus). Adv. Anim. Vet. Sci. 7(2): 73-81.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.2.73.81
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Al-Saffar and Almayahi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
It is well known, the vagina is that part of the reproductive duct situated between the uterine cervix cranially and the vestibule caudally and entirely positioned within the pelvic cavity. The vagina serves as the copulatory organ of the female that receives the male penis during copulation and acts as a passage way for the foetus during parturition (Budras and Habel, 2003). It could also be used to diagnose some general and reproductive diseases and abnormalities (Menzies, 2010; Goncagul et al., 2012; Yotov et al., 2013). Recently, Abiaezute and Nwaogu (2015) recorded postnatal developmental changes in the length and weight of the vagina of the female West African dwarf goat. Bal and Getty (1972) previously described changes with maturity in the vaginal histological structures of the domestic pig. In the female guinea pig, Cooper and Schiller (1975) recorded that the end of the reproductive tract is lack of vestibule and the vagina was ended by the external vaginal orifice. This orifice was U-shaped and was closed by a vaginal closure membrane.
Up to date there are no available studies in the previous and present literatures investigated the developmental histological changes in the pelvic parts of the reproductive organs of the female reproductive system of the local rabbit. According to that the study was performed due to the importance of this animal species as a model in many scientific experiments.
MATERIALS AND METHODS
The study was conducted during the period extended from March 2017 to April 2018. It was conducted under the order no. 310/ H. S. of the ethic committee of the council of the Veterinary Medicine College/Baghdad University on 5/Feb/2017. Twenty four female rabbits (Oryctolagus cuniculus) of three different ages were selected to conduct the current project. Apparent healthy rabbits were purchased directly from local rabbit’s breeders. The collected animals were one week newly born female kittens, 8 - 10 weeks aged immature does and mature does of five months (virgin). Animals were left three days under supervision to insure their good healthy condition during such period before their euthanasia and subsequent dissecting them.
Each rabbit was weighed with a sensitive weighing balance and euthanized by intramuscular injection of sodium pentobarbitone (140 mg/kg BW). The rabbits were placed on the dorsal recumbency to view their ventral aspect, thereafter; a mid-line abdominal incision was made to expose the peritoneal cavity structures. Subsequently, the pelvic symphysis was cut to view the pelvic organs of the reproductive system which were exposed and photographed in situ and later dissected out. The organs were temporarily kept in glass container to keep them moist in physiological saline solution. Then after each organ was transfer to filter paper to dry before weighing. After their extirpation on board, the weight and length were measured using a sensitive electronic balance with a sensitivity of 0.0001 g and vernier callipers, respectively. The data obtained expressed as mean ± standard error of the mean (mean ± SEM). Values of p < 0.05 were considered significant. All measurements were listed in table.
The vagina, vestibule and vulva as a whole were dissected and washed with normal saline and then by 10% neutral buffered formalin and eventually immersed in 10% neutral buffered formalin for 72 hrs. For future staining with histochemical stains, some specimens were fixed by Bouin’s solution.Next to fixation, specimens were dehydrated through ascending series of ethyl alcohol (70%, 80%, 90% and 100%) each for 2 hrs , then cleared with xylene for 15 mint. Specimens were infiltrated with paraffin wax (58 – 60 ºC) then embedded with paraffin wax to obtain blocks of paraffin. Paraffin sections of six microns were obtained by using rotary microtome. General and special stains were used to stains the tissue sections such as hematoxyline-eosin (H&E), Masson Trichrome (MTC), Gomori Trichrome, Alcine blue (AB) (pH 2.5), Periodic acid schiff (PAS) (Culling, 1985).
The thicknesses of tunica mucosa and muscularis in the vagina were measured using the colour USB 2.0 digital image system (Scope Image 9.0) which is provided with image processing software. All above parameters were set in table to achieve comparison among different postnatal ages. Tissues sections of 6 µm thick were stained by Gomori Trichrome stain because this stain facilitates the identification of the different tissues when carrying out micromorphometric measurements (Wei et al., 2011).
Statistical Analysis
All data of both macromorphometric and micromorphometric measurements were analyzed by one way ANOVA using SPSS software (version 14).
RESULTS
Macroscopic Findings
Gross examination of the female genital system of kittens, immature rabbits and five months aged virgin mature does revealed that it comprised of intra-pelvic organs such as vagina and vestibule as well as the external vulva (Figures 1, 2 & 3). The vagina in the local rabbits was characteristically very long tube-like structure. The organ entirely was running ventrally to the rectum together with vestibule in the pelvic cavity. Longitudinal incision in these two tubal organs revealed marked entrance of the pelvic urethra separating them from each other.
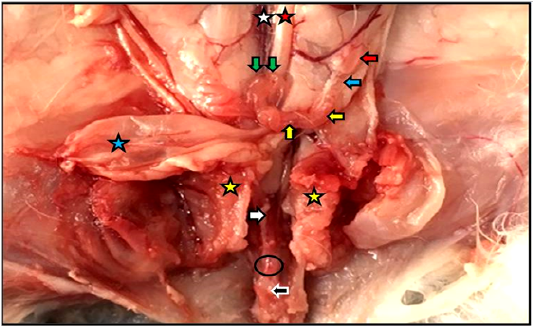
Figure 1: Reproductive system of the female kitten in situ showed vagina (white arrow) and vestibule (black arrow) inside the pelvic cavity, cut of pelvic symphysis (yellow stars), site of urethra cut (black circle) as well as ovary (red arrow), uterine tube (blue arrow), uterine horn (yellow arrows), cervices (green arrows), urinary bladder (blue star), abdominal aorta (red star), caudal vena cava (white star).
The means lengths and weights of the vagina in one week aged kittens were 8.0 ± 0.22 mm and 7.0 ± 0.05 mg, respectively, whereas, they were 39.0 ± 0.11 mm and 80.0 ± 0.07mg in immature does. Same measurements in mature does were 83.0 ± 0.03 mm and 360.0 ± 0.26 mg, respectively (Table 1).
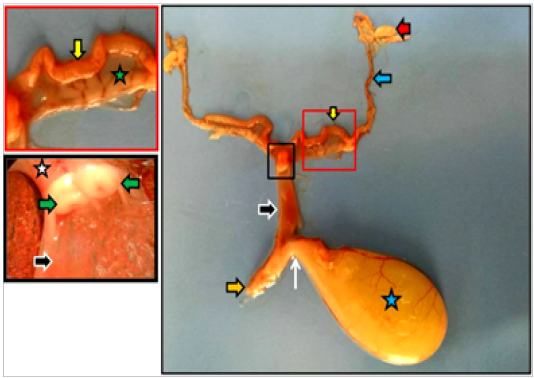
Figure 2: Dissected reproductive system of immature doe showed vagina (black arrows), vestibule (orange arrow), urethra (white arrow) as well as ovary (red arrow), uterine tube (blue arrow), uterine horn (yellow arrows), urinary bladder (blue star), cervices (white star), portio vaginalis uteri (green arrows).
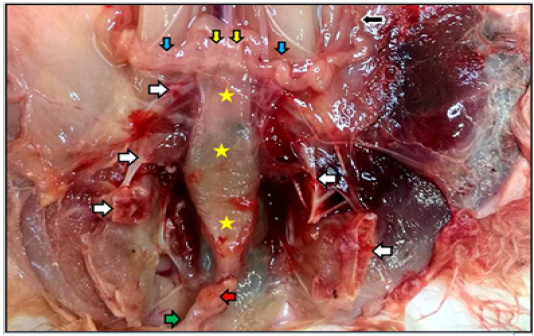
Figure 3: Reproductive system of the mature doe in situ showed vagina (yellow stars), vestibule (green arrow) inside the pelvic cavity, cut of pelvic symphysis (white arrows), site of urethra cut (red arrow) as well as uterine tube (black arrow), uterine horns (blue arrows), and cervices (yellow arrows).
The vestibule canal which was running ventral to the end part of the rectum was ended by the vulva and the latter was located just ventral to the anus. The vulva covered by hairy and pigmented skin externally at the labia major, whereas, non hairy and not pigmented skin at labia minor. The latter enclosed the luminal cleft due to the absence of real opening at this site of the reproductive tract.
The means of lengths and weights of the vestibules in one week aged kittens were 5.0 ± 0.01 mm and 3.0 ± 0.71 mg, respectively, whereas, they were 22.2 ± 0.33 mm and 40.0 ± 0.10 mg in immature does. Same measurements in mature does were 30.0 ± 0.02 mm and 80.0 ± 0.11 mg, respectively (Table 1).
Table 1: Gross measurements of intra-pelvic organs of the reproductive tract of the local rabbits at different ages
| Organs | Different aged rabbits |
Measurements
|
|
| Length (mm) | Weight (mg) | ||
| Vagina | Kittens | 8.0 ± 0.22 | 7.0 ± 0.05 |
| Immature does | 39.0 ± 0.11 | 80.0 ± 0.07 | |
| Mature does | 83.0 ± 0.03 | 360.0 ± 0.26 | |
| Vestibule | Kittens | 5.0 ± 0.01 | 3.0 ± 0.71 |
| Immature does | 22.2 ± 0.33 | 40.0 ± 0.10 | |
| Mature does | 30.0 ± 0.02 | 80.0 ± 0.11 | |
Microscopic Findings Vagina
Microscopic examination of the vagina wall in the three studied ages of female rabbits revealed three different regions that were cranial, middle and caudal regions. The cranial region was shorter part which surrounded the portio vaginalis of the cervix dorsally and fused with the cervix ventrally causing wrinkled internal mucosa of the vagina. Its diameter is smaller than the longest and widest middle region, while larger than the caudal shortest region that was ended at urethral orifice as a constricted part before the site of urethral opening between vagina and vestibule.
Vagina of Female Kittens
Microscopically, the wall of vagina in female kittens was composed of mucosa, muscularis and adventitia. In the cranial region, the findings showed that the mucosa was characterized by simple columnar lining epithelium under which thin lamina propria of loose connective tissue. Ventrally and closely adjacent to the portio vaginalis uteri, the mucosa showed the presence of short and small dome shaped projections. Tunica muscularis characterized by the presence of three differently oriented muscular bundles of smooth muscle fibers that were inner circular, middle longitudinal and outer circular muscle fibers. The tunica was thicker than the others such as mucosa and adventitia. The tunica adventitia was a thin layer of loose connective tissue characterized by the presence of autonomic sympathetic ganglia (Figure 4-A)
In the middle region of the vagina of the female kittens, the wall showed changes in the tunica mucosa. It revealed simple low columnar epithelial lining over thinner lamina propria than in the cranial part. The projections were short folds with rounded tips. Tunica muscularis showed similarly to the cranial regions three differently oriented muscular bundles of smooth muscle fibers but with prominently more thicken outer circularly oriented bundles (Figure 4-B).
In the caudal region of the vagina near the opening of urethra into the lumen of the beginning of vestibule, the wall was thinner than the other regions relatively. The mucosa showed ciliated pseudostratified columnar epithelial lining with very low unclear mucosal folds. The muscularis was thinner than the other regions and it was made mainly of circular bundles of smooth muscle fibers. Instead of that, tunica adventitia was thicker than other regions with the presence of many sympathetic ganglia.
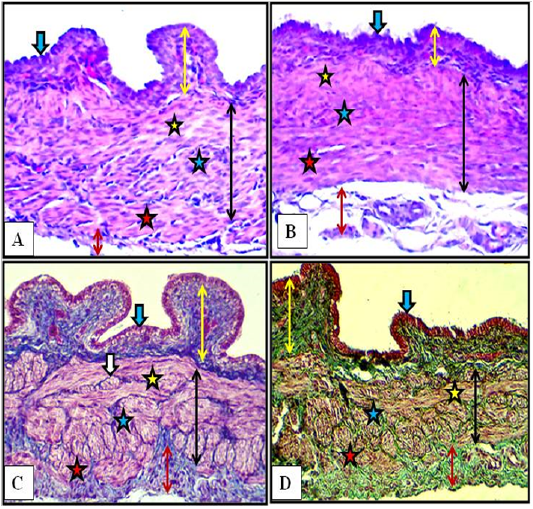
Figure 4: Cranial (A) and middle (B) regions of the vagina of the female kitten (upper panel) and cranial (C) and middle (D) regions of the vagina of the immature doe (lower panel) showed mucosa (double heads yellow arrows), simple columnar epithelium (blue arrow) and muscularis (double heads black arrows) of inner circular (yellow stars), middle longitudinal (blue stars) and outer circular (red stars), adventitia (double heads red arrow), nerve plexuses (white arrow). A & B: X200, H&E. C: X200, MTC. D: X200, Gomori Trichrome
Vagina of Immature Does
Microscopic findings in the cranial region of the vaginal wall of immature does revealed simple columnar epithelial lining with node-like projections present in the ventral area in close adjacent to the portio vaginalis uteri. Lamina propria was wider than that of the same region of the female kittens which was made up of loose connective tissue. Due to the well separating connective tissue fibers, the tunica muscularis was made of three obvious different layers of muscular bundles that were inner circular, middle longitudinal and outer circular muscle fibers. Nerve plexuses were identified in this connective tissue present betweenthese muscular layers. Inner circular layer was very thin, whereas, the middle longitudinal was thicker relatively. Thin tunica adventitia was formed of loose connective tissue rich in blood capillaries with the presence of sympathetic ganglia (Figure 4-C).
The middle region of the vaginal wall of the immature does revealed simple low columnar epithelial lining with small and short folds with rounded tips. Tunica muscularis was made of same layers of the smooth muscle fibers of the cranial region but the tunica appeared thinner in thickness (Figure 4-D).
In the caudal region of the vaginal wall of the immature does, the histological findings revealed pseudostratified columnar epithelial lining with the absence of folds. Tunica muscularis was made of only circular layer of smooth muscle bundles. Tunica adventitia was very thick compared to those of mucosa and muscularis. It was made of loose connective tissue rich blood vessels and many dispersed sympathetic ganglia (Figure 5).
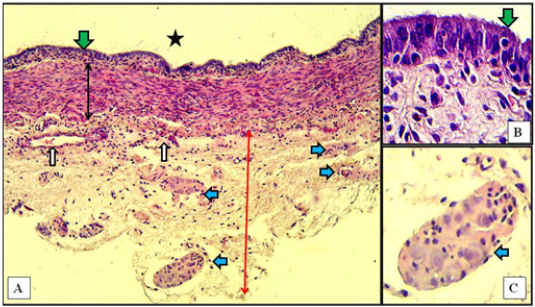
Figure 5: Caudal region of vagina of immature doe showed mucosa (green arrows) of pseudostratified columnar epithelium, muscularis (double heads black arrow), adventitia (red arrow), sympathetic ganglia (blue arrows), blood vessels (white arrows), and vaginal lumen (black star). A: X10, H&E. B: X100, H&E. C: X40, H&E
Vagina of Mature Does
The cranial region of the vaginal wall of mature does revealed simple columnar epithelial lining with tall and thin projections present in the ventral area in close adjacent to the portio vaginalis uteri. The underlying lamina propria was widest compared to those same regions in kittens and immature does. Tunica muscularis was made of inner thin layer of longitudinally oriented smooth muscle bundles and outer thick circularly oriented smooth muscle bundles. Tunica adventitia was prominently very thick compared to both tunica mucosa and muscularis which was made up of loose connective tissue filled with blood vessels and many dispersed sympathetic ganglia (Figure 6-A).
In the middle region of the vaginal wall of mature does, the epithelial lining was stratified columnar of 2 -3 cells in thickness with very low mucosal folds. Wide underlying lamina propria was found of loose connective tissue. Tunica muscularis was composed of thinner inner layer of longitudinally oriented smooth muscle bundles and thicker outer circularly oriented smooth muscle bundles. Tunica adventitia was very thick filled with blood vessels and sympathetic ganglia.
In the caudal region of the vaginal wall, histological findings were similar to those of the middle region but the stratified lining epithelium was thicker of more than three cellular layers. In addition to that the outer circular layer of the smooth muscle bundles was very thick, whereas the inner longitudinal layer was very thin. Tunica adventitia was as thick as the tunica muscularis. Characteristically, large sympathetic ganglion was detected adherent to the outer tunica adventitia in the ended part of the caudal region (Figure 6-B).
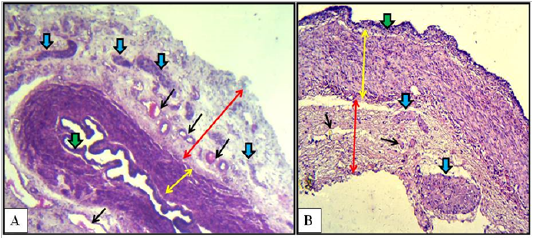
Figure 6: Cranial (A) and caudal (B) regions of the vagina of the mature doe showed mucosa (green arrows), muscularis (double heads yellow arrows), adventitia (double heads red arrows), sympathetic ganglia (blue arrows) and blood vessels (black arrows). A: X40, H&E. B: X100, H&E
Histochemical Staining of Vaginal Wall
The Masson Trichrome and Gomori Trichrome were applied successfully to stain the connective tissue structure present in the wall of the vagina of the three studied ages that were female kittens, immature does and mature does. The stains identified the collagenous fibers which was constituted the lamina propria underneath the lining epithelium of the vagina. Also they were clearly stained the connective tissue collagenous fibers separated the three differently oriented muscular bundles of the tunica muscularis that were inner longitudinal, middle circular and outer longitudinal bundles. The loose connective tissue constituents of the tunica adventitia also stained (Figures 4-C & D).
Micromorphometric Data of Vaginal Wall
Micromorphometric measurements of the vaginal wall thickness at different vaginal regions of the female kittens, immature and mature does were listed in Table 2. In general, the average vaginal wall thickness was 201.11 µm, 289.62 µm and 329.19 µm in female kittens, immature and mature does.
In the female kittens, the thicknesses of mucosa and muscularis were 25.84 µm and 196.45 µm at the cranial region, 33.54 µm and 169.32 µm at the middle region and at the caudal region were 57.97 µm and 119.22 µm. As a result, the total thickness of mucosa plus muscularis was decreased in the direction cranio –caudally which were 222.29 ± 0.21 SE, 203.86 ± 0.19 SE and 177.19 ± 0.11 SE at the cranial, middle and caudal regions, respectively.
Table 2: Micromorphometric measurements of the vaginal wall in female kittens, immature does and mature does
| Different ages of female rabbits | Regions | Mucosa (µm) | Muscularis (µm) | Total (µm) | Averages (µm) |
|
Kittens
|
Cranial | 25.84 | 196.45 | 222.29 ± 0.21 SE |
201.11 |
| Middle | 33.54 | 169.32 | 203.86 ± 0.19 SE | ||
| Caudal | 57.97 | 119.22 | 177.19 ± 0.11 SE | ||
|
Immature does |
Cranial | 41.34 | 279.42 | 320.76 ± 0.32 SE |
289.62 |
| Middle | 47.72 | 240.40 | 288.12 ± 0.22 SE | ||
| Caudal | 60.70 | 199.30 | 260.00 ± 0.13 SE | ||
|
Mature does |
Cranial | 44.08 | 302.09 | 346.17 ± 0.45 SE |
329.19 |
| Middle | 54.15 | 281.82 | 335.97 ± 0.32 SE | ||
| Caudal | 67.18 | 238.26 |
305.44 ± 0.27 SE |
Notes:
* adventitia was excluded
* Data showed that the mucosa was increased in thickness in the direction cranio- caudally of the vagina in all studied ages of rabbits, whereas, in contrary, the thickness of tunica muscularis was decreased in the same direction.
* Significant increased (p<0.05) in the thickness of vaginal wall of the immature does compared to those of kittens.
* Significant increased (p<0.05) in the thickness of the vaginal wall of the mature does compared to those of immature does
In immature does, the thicknesses of mucosa and muscularis were 41.34 µm and 279.42 µm at the cranial region, 47.72 µm and 240.40 µm at the middle region and at the caudal region were 60.70 µm and 199.30 µm. According to these measurements, the total thickness of mucosa plus muscularis was decreased in the direction cranio – caudally which were 320.76 ± 0.32 SE, 288.12 ± 0.22 SE and 260.00 ± 0.13 SE at the cranial, middle and caudal regions, respectively.
In mature does, the thicknesses of mucosa and muscularis were 44.08 µm and 302.09 µm at the cranial region, 54.15 µm and 281.82 µm at the middle region and at the caudal region were 67.18 µm and 238.26 µm. According to these measurements, the total thickness of mucosa plus muscularis was decreased in the direction cranio – caudally which were 346.17 ± 0.45 SE,335.97 ± 0.32 SE and 305.44 ± 0.27 SE at the cranial, middle and caudal regions, respectively.
Vestibule and Vulva
Microscopic examination of the vestibule showed similar histological structures in kittens, immature and mature does. Actually, there were no changes related to studied ages. As mentioned previously in the gross findings of the present results, the changes in this organ were just macromorphometric in nature. In another aspect, the microscopic picture showed slight changes between the cranial part and the caudal part of the vestibule in all ages of rabbits.
In kittens, the cranial part of the vestibule characterized by mucosa that lined with stratified squamous epithelium. That rest at lamina propria of loose connective tissue and invaded by blood vessels. Some of mucosal invaginations into the lamina propria were detected to develop subsequently into vestibular glands. The tubular structure surrounded by thin muscular layer of smooth muscles bundles arranged circularly. The thick loose connective tunica adventitia was invaded by numerous blood vessels (Figure 7-A). In the caudal part, same mucosa and mucosal invaginations were observed and the lamina propria filled with blood capillaries superficially and large vessels deeply with the presence of spars of circular smooth muscle fibers sunk into the surrounding connective tissue adventitia (Figure 7-B).
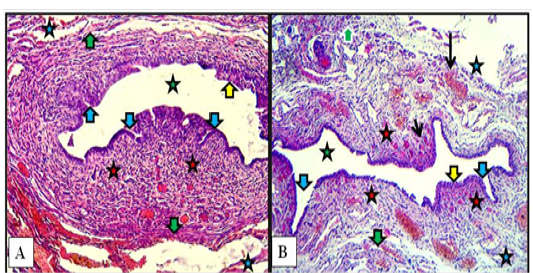
Figure 7: Cranial (A) and caudal (B) parts of vestibule of the female kitten showed mucosa of stratified squamous epithelium (yellow arrows), mucosal invaginations to form vestibular glands (blue arrows), lamina propria of loose connective tissue filled with blood vessels (red stars), superficial blood capillaries (short black arrow) and large vessels deeply (long black arrow), thin circular muscularis (green arrows), adventitia (blue stars) and lumen of vestibule (green stars). A & B: X100, H&E.
In immature does, similar vestibular wall structures were found with slight differences. In the cranial part nearby the urethral opening the circular smooth muscle bundles were more prominent than in that of kittens. In the cranial and caudal parts, some of newly formed vestibular glands were recorded (Figure 8).
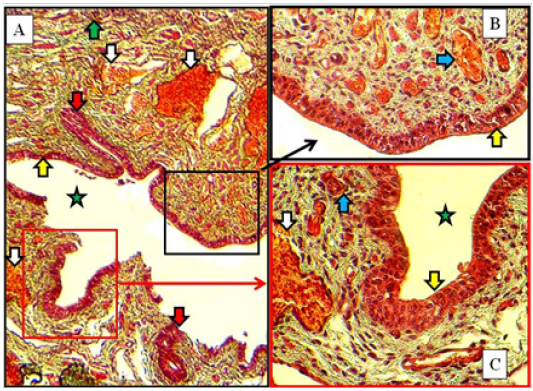
Figure 8: Caudal part of vestibule of immature doe showed stratified squamous epithelium (yellow arrows), lamina propria filled with blood capillaries superficially (blue arrows) and large vessels deeply (white arrows), vestibular glands (red arrow) and lumen (green star). A: X100. B & C: X400, MTC
In mature does, same structures as in immature does but the vestibular glands were increased in number and extended deeply into the lamina propria in both cranial and caudal parts of vestibule. The large blood vessels were prominently observed deeply in lamina propria adjacent to the thin muscularis of smooth muscle bundles (Figure 9).
The, Masson Trichrome and Gomori Trichrome were applied to stain the connective tissue structure present in the wall of the vestibule of the female kittens, immature does and mature does. The stains characterized as well the collagenous fibers which was constituted the lamina propria underneath the lining epithelium of the vestibule. Also they were clearly stained the connective tissue collagenous fibers interspersed between the muscular bundles of the tunica muscularis. The stains also stained the wide loose connective tissue constituents of the tunica adventitia (Figures 8 & 9).
The microscopic examination of the vulva showed no age related changes. Serial cross sections showed that this last part of the reproductive tract was lined with non keratinized stratified squamous epithelium that changed at the labia major into keratinized and pigmented with the presence of hairs bulbs. Thin layer of loose connective tissue under such epithelium was found. The surrounding wall to the vulvar cleft was made of strands of fine smooth muscle fibers rich by cavernous blood vessels. The smooth muscle strands arranged as inner longitudinally and outer circularly fibers (Figure 10).
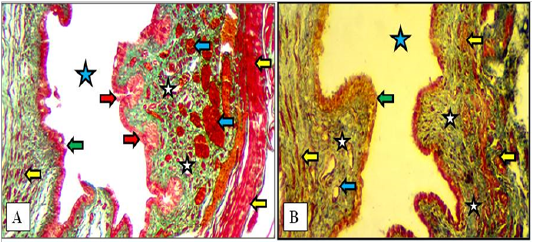
Figure 9: Cranial (A) and caudal (B) parts of the vestibule of the mature doe showed mucosa of stratified squamous epithelium (green arrows), lamina propria of loose connective tissue (white stars) filled with blood vessels (blue arrows), newly formed vestibular glands (red arrows), thin circular muscularis (yellow arrow) and lumen of vestibule (blue stars). A: X100, Gomori Trichrome. B: X200, MTC
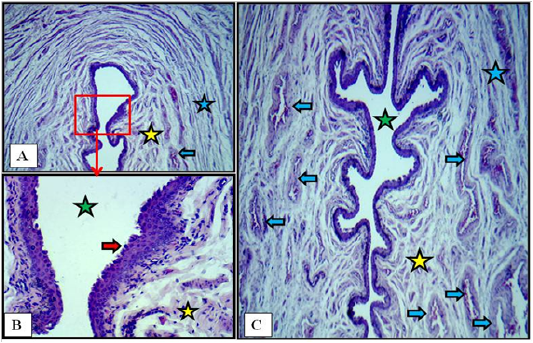
Figure 10: Cross section at the labia minor of vulva of immature doe showed vulvar cleft (green stars), stratified squamous epithelium (red arrows), cavernous vessels (blue arrows), inner longitudinal (yellow stars) and outer circular smooth muscle fibers (blue stars). A: X40. B: X400. C: X40. H&E
DISCUSSION
Microscopic findings regarding the wall of the vagina revealed characteristic features in all studies ages of the local female rabbits. In addition to its distinct very long length compared to other laboratory species as well as other mammalian species, the wall was heavily supplied with sympathetic ganglia existed in a thick vaginal adventitia. These structures were identified even in the vagina of female kittens but certainly were more advanced and prominently recognized in immature and mature does. Nerve plexuses were well identified in immature and subsequent mature does in their tunica muscularis intervening between the muscular bundles.
The lining epithelium ranged from simple columnar (cranially) to pseudostratified columnar or stratified squamous non keratinized (caudally) which gave negative reactions toward the histochemical staining procedures. The vaginal lining epithelium was different at different three parts of the rabbit vagina and in another aspect there were differences between kittens and the other ages such as immature and mature does. These findings were in a good agreement with previous studies stated that the lining epithelium of the cranial region of the vagina of adult animals was different than that of the caudal region (Awad et al., 1982). Additionally, the lining epithelium of the developing proper vagina was different than the vagina of adult (El-hariri et al., 1988; Farouk et al., 2012).
The thick musculature cranially became thinner caudally near the vestibule and so the three muscular layers of muscularis were subsided to become only one circular layer. Oppositely, the adventitia became thicker caudally and filled with blood vessels and ganglia. The presence of the three distinct tunicae in the vagina of local rabbits was in accordance with other mammalian species (Tortereau et al., 2013; Singh et al., 2014; Oh et al., 2014; Abiaezute and Nwaogu, 2015; Lalithamma et al., 2016).
The present microscopic data showed that the vaginal wall of female rabbits was constructed of three structural tunicae that were mucosa, muscularis and adventitia in which the mucosa was stratified squamous non keratinized and absence of the glands in the underlying lamina propria and such findings were in a good agreement with histological records of Junqueira et al. (2005).
In fact, the mucosa in the local does showed absence of vaginal glands because it is affected by the hormones changing caused by different stages of estrous cycle. Lamina propria exhibited very rich vascularization which may act as a source of fluid exudates which oozed through the epithelium during sexual stimulation. These findings came in accordance with previous postulations of Gu et al. (2005) that were the effects of ovarian hormones on the epithelium and the subjacent connective tissue lamina propria as well as the wall musculature of the reproductive tract. Noakes et al. (2001) mentioned that the vaginal epithelium in all adult mammals is hormone dependent in which the height and degree of keratinisation of the epithelium will be varied with the various stages of the estrous cycle.
The distinct nerve plexuses and sympathetic ganglia in the vagina of the studied female rabbits give attention to their role and function during coitus which may be achieved by reflex and sending stimuli to the central nervous system that may stimulate pars distalis to secrete the gonadotrophic hormones so that induced the ovulation. In fact, Brauera and Smith (2015) reviewed recently that the female reproductive tract is provided well with a rich ground plexus of autonomic nerves by which can regulate many functions such as vascular and non vascular smooth muscle contractile activities, glandular secretions and communicate the information with the central nervous system.
Current data showed absence of great age related changes in the microscopic structures of both vestibule and vulva. As mentioned in the results the vestibule was prominently very long so that the histological appearance was slightly changed in the direction from the vagina cranially to the end of the reproductive tract caudally. Similarly to other species that have vestibule, the vestibule wall in the local rabbits was constructed of three layers that were mucosa, muscularis and adventitia with the presence of very rich blood supply. That accordance with description of Samuelson (2007) as the mammalian vestibule contains large numbers of blood vessels where it formed venosus and cavernous erectile tissues. In another aspect, vestibular lining epithelium in the local rabbits was non keratinized stratified squamous epithelium and such finding agreed well with previous records (Chhieng and Hui, 2010; Emam et al., 2013).
CONCLUSION
Current study recorded differences in both macroscopic and microscopic as well as morphometric aspects of the vagina of the local rabbits compared to other animal species, specifically the species belong to rodent’s family. The current study believed that the vagina in the local does play a role in the stimulation of hypothalamus which controls the pars distalis of pituitary to produce profuse gonadotrophic hormones at coitus. The vagina as well as the vestibule was characteristically very long in length and their walls were heavily supplied with sympathetic ganglia. The hypothesis is that in mature does the neuro-hormonal action will takes place during mating and subsequent ovulation.
The study findings were in agreement and confirmed previous records and postulations of Al-Saffar and Al-Hasnawy (2014) and Al-Saffar and Al-Haik (2016a, 2016b, 2017a, 2017b) that rabbits were different from rodents in their organs anatomically, histologically and histochemically. Unique differences in the present records in the local rabbits confirmed the reason by which rabbits were separated and classified in new different order in the animal classification or taxonomy. In fact, rabbits are unique animal species and they have their own species variations and that’s why they were separated from rodent in order Rodentia in the beginning of the past century.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors strongly acknowledge the council of the Veterinary Medicine College / Baghdad University to support this research project.
AUTHOR’s CONTRIBUTION
AL-Saffar suggested the project, conducted the dissection of animals, aid in histological technique and preparation of histological sections, made gross and microscopic photography, described the microscopic results and revised the manuscript.
Almayahi aid in the dissection of the animals of the study. She processed the histological steps, stains the prepared slide histological sections, watch and took care about the animal’s health during the whole period of the project steps. She described the gross morphology aspect of the results, aid in the photography and made all of the macromorphometric and micromorphometric analysis.
References






