Advances in Animal and Veterinary Sciences
Review Article
Salmonella and Listeria monocytogenes: A Review of Prevalence and Antibiotic Resistance in Chickens and their Processing Environments
Mustapha Goni Abatcha
Food Technology Division, School of Industrial Technology, Universiti Sains Malaysia, 11800 Minden, Penang, Malaysia.
Abstract | Salmonellosis and listeriosis represent important foodborne diseases that continue to pose major challenges to national economic and public health, worldwide. Currently, the leading source of human infection is considered to be due to consumption of contaminated raw or undercooked poultry meat and their products. However, exploration of major databases revealed that Salmonella and Listeria monocytogenes have been identified in the environment, humans, and food animals, worldwide. This review highlights the prevalence and antibiotic resistance of Salmonella and Listeria monocytogenes in chicken and their related processing environments. There is a difference in the prevalence of these pathogens and their resistance to a wide range of antibiotics. The overall prevalence of Salmonella spp. and Listeria monocytogenes was 39.0% (range, 4.0%–88.46%) and 25.2% (range, 2.5%–60%), respectively. Likewise, in this survey, a high percentage of Salmonella isolates were resistant to erythromycin (89.7%), sulphonamide (63.6%), tetracycline (62.2%), ampicillin (58.0%), streptomycin (46.7%), and nalidixic acid (46.1%). The L. monocytogenes were most resistant to nalidixic acid (100%), oxacillin (90.0%), clindamycin (59.5%), and ceftriaxone (59.0%). Such baseline information is essential for use in developing effective risk management strategies of foodborne pathogens in chicken and poultry products.
Keywords | Salmonella spp., Listeria monocytogenes, Prevalence, Chicken, Environment
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 05, 2017; Accepted | August 18, 2017; Published | September 17, 2017
*Correspondence | Mustapha Goni Abatcha, Food Technology Division, School of Industrial Technology, Universiti Sains Malaysia, 11800 Minden, Penang, Malaysia; Email: [email protected]
Citation | Abatcha MG (2017). Salmonella and listeria monocytogenes: A review of prevalence and antibiotic resistance in chickens and their processing environments. Adv. Anim. Vet. Sci. 5(9): 377-385.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.9.377.385
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Abatcha M.G. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
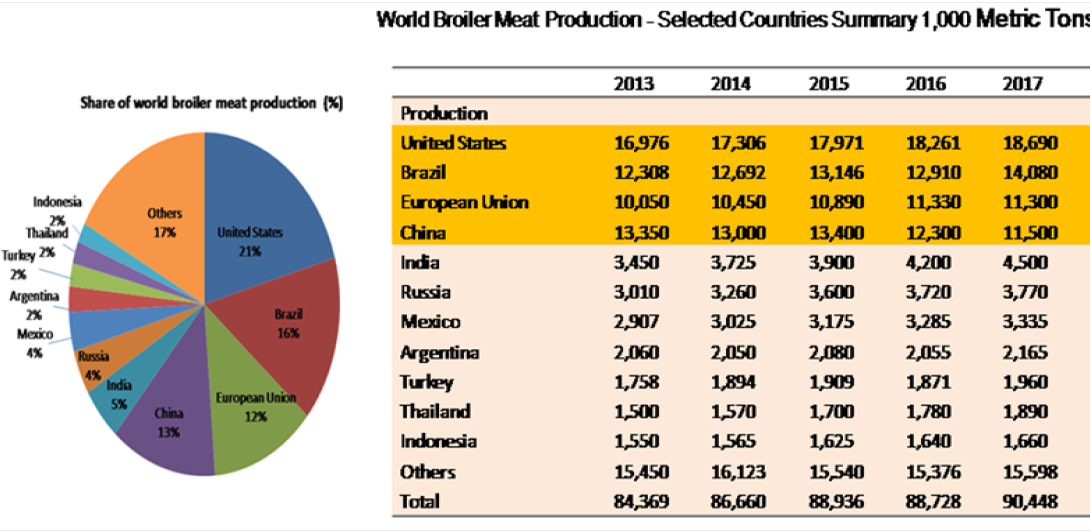
Poultry comprises chicken, turkey, duck, and laying hens; however, chicken (Gallus gallus) is the main species used for meat production, representing approximately 88% of global poultry meat output and hence, providing an affordable source of protein to human (Skarp et al., 2016). Global broiler meat production has increased from 84.3 million tonnes in 2013 to 90.3 million tonnes in 2017 (USDA, 2017).The largest producer of broiler meat is United States (21% of world production), followed by Brazil (16%), China (13%), European Union (12%), India (5%), and Russia and Mexico (4% each) (Figure 1). The growth in demand for poultry meat without the infrastructure for appropriate sanitary handling may lead to the transmission of pathogenic microorganisms from animals to the consumer (Soomro et al., 2011).
Contamination of poultry meat with foodborne pathogens remains an important economic and health concern worldwide because it can lead to human illnesses and loss of productivity due to absenteeism, adding significant costs to production and healthcare (White et al., 2002; Mead 2004). According to a published CDC data, in the US alone, foodborne diseases account for an estimated 76 million illnesses, 325,000 hospitalisations, and 5,000 deaths annually. Among foodborne pathogens, nontyphoidal Salmonella spp. and L. monocytogenes were responsible for 47% of the reported deaths in 2011 (CDC, 2011). Salmonella and L. monocytogenes are major foodborne bacterial pathogens found in raw poultry meat products and in the processing environment (Carrasco et al., 2012; Foley and Lynne, 2008). Poultry has been a potential vehicle for L. monocytogenes and Salmonella spp. transmission (Ryser and Marth, 2007). The main concerns are during processing; certain stages including scalding, de-feathering, evisceration, and chilling of carcasses have been documented as possible cross-contamination pathways to others and processing environments (Todd and Notermans, 2011). As a result, many countries have adopted a stringent policy concerning the presence of foodborne pathogens such as Salmonella spp. and Listeria monocytogenes in foods (Yue, 2014).
The emergence of antimicrobial-resistant foodborne pathogens is a major health problem. The use of antimicrobials in food animals for growth promotion, prophylaxis, and treatment of poultry diseases has increased. However, the overuse of these antibiotics may increase the rates of antimicrobial resistance to several antibiotics (Castanon, 2007; Mathew et al., 2007). Over the years, food borne pathogens are continuously becoming resistant to regularly-used antibiotics, and this causes an added difficulty for people with these infections because salmonellosis and listeriosis require appropriate antimicrobial therapy. In this survey, we summarised the current reports on the prevalence and antimicrobial resistance of Salmonella spp. and L. monocytogenes in chickens and their processing environment.
MATERIAL AND METHODS
Published articles collection on prevalence and antibiotic resistance of Salmonella spp. and L. monocytogenes in chickens and processing environment was retrieved from Thomson Reuters Web of Science. Also, other databases were equally used including Science Direct, Scopus, Springer, Wiley, and PubMed using the words “Salmonella, and chickens” and “L. monocytogenes and chickens”. The search was extended to other open-access journals that contain articles on the subject matter. All the articles used were publications up to date.
RESULTS AND DISCUSSION
Prevalence and Antibiotic Resistance of Salmonella in Chickens and their Processing Environment
The prevalence of Salmonella spp. among several studies was profoundly variable (Table 1). The prevalence varied from 4.0% to as high as 88.46%. Countries such as South Africa and Malaysia reported a higher prevalence of ≥ 60%. In Malaysia, a higher prevalence of Salmonella spp. in chicken carcasses and their related processing environment in wet market and processing plant (88.46%) was reported (Nidaullah et al., 2017). Similarly, Modarressi and Thong (2010) recorded a high prevalence of Salmonella (72.7%) in chicken meat sample in Kuala Lumpur. Meanwhile, in South Africa, Van Nierop et al. (2005) reported that 60.6% chicken carcasses samples were contaminated with Salmonella spp. The relatively high contamination rate suggests that chicken carcass or meat may be a potential vehicle for the transmission of Salmonella species. Also, the cross-contamination of Salmonella could occur during handling, processing, packing, and distribution (Uyttendaele et al., 1998).
Table 1: Prevalence (%) of Salmonella species in chicken and their processing environment
|
Country |
Samples |
Serotypes |
Prevalence (%) |
References |
|
Spain |
Retail Chicken meat |
S. Enteritidis, S. Hadar, S.4,12:b:-(II), S. Mbandaka, S. Derby, S. Virchow and S. Paratyphi B |
35.83 |
Domınguez et al., (2002) |
|
Chicken carcasses |
S. Enteritidis, S. Poona, S. Paratyphi Band S. Worthington |
49.0 |
Capita et al., (2003) |
|
|
Albania |
Chicken meat |
S. Enteritidis, S. Senftenberg, S. Newport, S. Albony, S. Agona, S.Banana, S. Brancaster, S.Infantis and S. Oslo |
6.5 |
Beli et al., (2001) |
|
Belgium |
Poultry carcasses and product |
S. Hadar, S. Enteritidis, S. Infantis, S. Virchow, S. Indiana, S. Typhimurium, S. Heidelberg, S. London, S. Newport, S. Agona, S. Braenderup, S. Brandenburg, S. Muenchen, S. Coeln, S. Derby, S. Livingstone, S. Shwarzengrund, S. sp |
36.5 |
Uyttendaele et al., (1999) |
|
Brazil |
Chicken Carcass and processing environmental |
S. Enteritidis, S. Albany, S. Hadar, S. Indiana, S. I 4,12:z:– (8%), S. Bredeney, S. Braenderup, S. Typhimurium, S. Mbandaka, S. Saint Paul |
41.0 |
Fuzihara et al., (2000) |
|
Korea |
Chicken meat |
S. Agona, S. Dessau, S. Montevideo, S. Lome (Vi+) S. Enteritidis, S. Weltevreden |
42.3 |
Hyeon et al. (2011) |
|
UK |
Whole Raw Chicken |
S. Hadar, S. Enteritidis and S. Indiana, S. Vircho, S. Heidelberg, S. Agona, S. Anatum, S. Bredeney and S. Typhimurium, S. Infantis, S. Kentucky, S. Livingstone, S. Newport and S. Worthington |
25.0 |
Jørgensen et al. (2002) |
|
USA |
Chicken Carcass and processing environmental |
S. Senftenberg, S. Thompson, S. Montevideo, S. Braenderup, S. Derby, S. Hadar, S. Infantis, S. Kentucky, S. Mbandaka, S. Muenchen, S. Ohio, S. Agona, S. Brandenberg, S.Enteritidis, S.Typhimurium, S. Copenhagen, S. Schwarzengrund, S. Heidelberg |
6.1 |
Bailey et al., (2001) |
|
Saudi Arabia |
Poultry and processing environmental |
S. Enteritidis, S. Virchow, S. Paratyphi B var. Java, S. Infantis, S. Infantis, S. Livingstone, S. Typhimurium, S. Sofia, S. Amersfoort, S. Senftenberg, S. Gaminara, S. Indiana, S. Kingston, S. Amersfoort, S. Virchow, S. Thompson, S. Altona, S. Tarshyne and S. Anatum. |
4.0 |
Al-Nakhli et al., (1999) |
|
South Africa |
Chicken carcasses |
S. Hadar, S. Blockley, S. Irumu, S. Snatum, S. Reading, S. Virchow, S. Schwarzengrund, S. Westhampton, S. Typhimurium, S. Derby, S. Heidelberg |
60.6 |
Van Nierop et al., (2005) |
|
Malaysia |
Chicken carcasses and processing environmental |
S. Albany, S. Corvallis, S. Brancaster S. Enteritidis, S. Typhimurium, S. Florian, S. Braenderup, S.Give, S. Weltevreden, S. Kivu, S. Sarajane, S. Haifa, S. Indiana, S. Kentucky, S. Oyonnax, S. Chester and Salmonella Stanley |
88.46 |
Nidaullah et al., (2017) |
|
Chicken Meats |
S. Corvallis, S.Typhimurium, S. Hadar, S. Enteritidis, S.Weltevreden, S.Agona, S.Newport, S.Albany, S. Istanbul, S. Emek and S.Wandsworth |
72.7 |
Modarressi and Thong (2010) |
Furthermore, the prevalence rate varies substantially in some countries (Table 1). A prevalence rate of 49.0% was found in chicken carcasses in Spain. This was followed by poultry carcasses and product in Belgium (36.5%), retail chicken meat in Spain (35.83%), whole raw chicken in the UK (25.0%), and chicken meat from Albania (6.5%). In Korea, Brazil, and the United States, the prevalence of Salmonella spp. ranged from 6.1% to 42.3%. In contrast, the Saudi Arabia recorded a lower prevalence of 4.0% for poultry and its related processing environments (Al-Nakhli et al., 1999). This could be partly due to differences in detection methods used, sampling strategy, and sample type. Over the years, the prevalence of Salmonella has drastically increased worldwide, including Malaysia. This may possibly be connected to increased poultry production and the stress induced during poultry transportation (Arumugaswamy et al., 1995), whereas in wet markets, the high prevalence of Salmonella can be attributed to poor sanitation and
Table 2: Prevalence (%) of Antimicrobial Resistance Salmonella among chicken and their processing environment
|
Antimicrobial agent |
Prevalence (%), Medeiros et al., (2011) |
Prevalence (%), Al-Zenki et al., (2007) |
Prevalence (%), Parveen et al. (2007) |
Prevalence (%), Yildirim et al. (2011) |
Prevalence (%), Thong and Modarressi (2011) |
Overall (%) Prevalence |
|
Ampicillin |
38.0 |
97.1 |
52.9 |
85.2 |
17.0 |
58.0 |
|
Aztreonam |
19.2 |
- |
- |
- |
- |
19.2 |
|
Cephalotin |
12.0 |
2.9 |
- |
52.9 |
8.0 |
18.95 |
|
Cefoxitine |
13.2 |
- |
52.0 |
- |
2.2 |
22.5 |
|
Ceftriaxone |
6.0 |
- |
0.0 |
- |
0.0 |
2.0 |
|
Ceftiofur |
28.0 |
0.0 |
51.7 |
- |
0.0 |
19.9 |
|
Florfenico |
62.0 |
1.2 |
- |
- |
31.6 |
|
|
Chloramphenicol |
6.0 |
1.2 |
0.0 |
10.2 |
10.2 |
5.52 |
|
Streptomycin |
78.0 |
0.6 |
35.2 |
61.7 |
57.9 |
46.7 |
|
Gentamicin |
12.0 |
4.0 |
0.7 |
14.7 |
2.2 |
6.72 |
|
Nalidixic acid |
40.0 |
100 |
0.0 |
- |
44.3 |
46.1 |
|
Ciprofloxacin |
4.0 |
0.6 |
0.0 |
- |
2.2 |
1.7 |
|
Enrofloxacin |
19.2 |
- |
- |
- |
- |
19.2 |
|
Tetracycline |
12.0 |
96.5 |
73.4 |
67.6 |
73.8 |
62.2 |
|
Sulfonamide |
58.0 |
- |
- |
- |
63.6 |
63.6 |
|
Trimethoprim |
10.0 |
4.0 |
- |
- |
- |
7.0 |
|
Trimethoprim-sulfamethoxazole |
10.0 |
2.3 |
0.0 |
- |
19.3 |
7.9 |
|
Nitrofurantoin |
8.0 |
- |
- |
- |
- |
8.0 |
|
Spectinomycin |
- |
1.2 |
- |
- |
- |
1.2 |
|
Amoxicilline-clavulanic acid |
- |
0.6 |
52.0 |
- |
1.4 |
18.0 |
|
Apramycin |
- |
0.6 |
- |
- |
- |
0.6 |
|
Neomycin |
- |
0.6 |
- |
55.8 |
- |
28.2 |
|
Colistin |
- |
0.0 |
- |
- |
- |
0.0 |
|
Sulfisoxazole |
- |
- |
21.8 |
- |
- |
21.8 |
|
Kanamycin |
- |
- |
6.3 |
- |
6.8 |
6.55 |
|
Amikacin |
- |
- |
0.0 |
2.9 |
1.0 |
1.3 |
|
Erythromycin |
- |
- |
- |
89.7 |
- |
89.7 |
|
Cefotaxime |
2.9 |
0.0 |
1.45 |
|||
|
Ceftazidime |
- |
- |
- |
- |
0.0 |
0.0 |
NB: —, not done.
hygiene practices. The occurrence of Salmonella in poultry at the farm level might be minimal but transportation of live birds in overcrowded cages over a long distance will lead to stress among the poultry, thereby causing an increase in shedding of Salmonella (Marin and Lainez, 2009; Heyndrickx et al., 2002). In Canada, Arsenault et al. (2007) observed that the prevalence of Salmonella-positive flocks is around 50%. When poultry flocks are infected at farms, Salmonella is carried asymptomatically in the gastrointestinal tract and can be readily transferred to carcasses through faecal contamination in the abattoir (Carrasco et al., 2012). Also, the role of feed components as a cause of Salmonella in the poultry industry has drawn great consideration (Bailey et al., 2001). Feed products may constitute a risk factor for introducing Salmonella since they are made from a wide range of potentially contaminated ingredients (Crump et al., 2002; Jiang, 2016).
The distribution of Salmonella serovars differs over time due to various geographical locations, production scale, and the country’s development status (Hendriksen et al., 2011). In this review, some Salmonella serovars were reported to be predominant in many countries. For instance, S. Enteritidis and S. Hadar were found to be the major Salmonella serovars in Spain by Domınguez et al. (2002), in Belgium by Uyttendaele et al. (1999), in Brazil by Fuzihara et al. (2000), in the UK by Jørgensen et al. (2002), and in Malaysia by Modarressi and Thong (2010), respectively.
Table 3: Prevalence (%) of Listeria monocytogenes in chicken and their processing environment
|
Country |
Year |
Samples |
Prevalence(%) |
References |
|
China |
2007 |
Raw meat |
7.7 |
Chen et at., 2009 |
|
Poland |
2004 |
Raw chicken |
7.14 |
Kosek-Paszkowska et al., 2005 |
|
UK |
1998 |
Raw chicken |
60.0 |
Pini and Gilbert (1988) |
|
Thailand |
2009 |
Frozen chicken meat |
2.50 |
Kanarat et al. (2011) |
|
Turkey |
2015 |
Chicken wing meat |
45.0 |
Elmali et al., (2015) |
|
Pakistan |
2003 |
Fresh chicken meat |
12.5 |
Mahmood et al.,(2003) |
|
Malaysia |
2012 |
Raw chicken |
20.0 |
Goh et al., (2012) |
|
Jordan |
2010 |
Fresh chicken |
9.4 |
Osaili et al., (2011) |
|
Brazil |
2004 |
Raw chicken breast |
40.0 |
Loura et al., (2005) |
|
Bangladesh |
2016 |
Fresh chicken |
8.33 |
Islam et al., (2016) |
|
France |
2001 |
Chicken processing environment |
22.1 |
Chasseignaux et al., (2001) |
|
UK |
1992 |
Chicken processing environment |
26.0 |
Lawrence and Gilmour (1994) |
|
France |
Chicken processing environment |
38.9 |
Chasseignaux et al., (2002) |
|
|
Algeria |
Chicken processing environment |
8.9 |
Bouayad et al., 2015 |
|
|
France |
1994 |
Chicken processing environment |
55.0 |
Salvat et al., (1995) |
|
Brazil |
2004 |
Chicken processing environment |
40.0 |
Loura et al., (2005) |
Moreover, a rapid international trade in agricultural products and food has eased the dissemination of Salmonella serovars across the international boundaries of importing countries (D’Aoust, 1994). On the other hand, S. Hadar has been targeted by European Union regulations as one of the five serovars to be reduced in prevalence in the European Union in poultry populations (EFSA, 2014). In Greece, S. Hadar ranked between 0.65% and 2.9% during 2007–2012 among human isolates (EFSA, 2014).
Antibiotic resistance of Salmonella spp. isolated from chickens is shown in Table 2. Antimicrobial resistance was exclusively varied with a particular study, location, and the sample examined. There is a notable variation in resistance of Salmonella spp. from chickens to a wide variety of antimicrobial agents in studies reported worldwide. In this survey, relatively high resistances occurred for erythromycin (89.7%), sulphonamide (63.6%), tetracycline (62.2%), ampicillin (58.0%), streptomycin (46.7%), nalidixic acid (46.1%) florfenicol (31.6%), neomycin (28.2%), cefoxitin (22.5%), sulfisoxazole (21.8%), ceftiofur (19.9%), aztreonam andenrofloxacin (19.2% each), cephalothin (18.95%), and amoxicillin-clavulanic acid (18.0%). Multidrug resistance has been reported in many serovars of Salmonella associated with poultry (Parveen et al., 2007; Al-Nakhli et al., 1999; Yildirim et al., 2011).The detection of multi-resistance in foodborne Salmonella isolates is a worrying health concern; there is a need for continuous surveillance and of more prudent use of antibiotics. In poultry, the emergence of Salmonella with antimicrobial resistance is mainly promoted by the use of antibiotics as a growth promoter in feed, prophylaxis, and therapeutics for treating bacterial infections (Hyeon et al., 2011). Although antibiotic use is under veterinary prescription control, farmers are still inclined to use antibiotics as prophylactic in intensive farming units, mainly poultry, cattle, and pigs (Usera et al., 2002). Likewise, in this survey, a small percentage of Salmonella spp. demonstrated lower resistance to nitrofurantoin, trimethoprim-sulfamethoxazole, trimethoprim, kanamycin, ceftriaxone, ciprofloxacin, spectinomycin, and apramycin. In contrast, 100% susceptibility to neomycin and ceftazidime was observed.
Prevalence and Antibiotic Resistance of Listeria Monocytogenes in Chickens and their Processing Environment
The prevalence of L. monocytogenes ranged from 2.5% to 60% in chicken meats and 8.9% to 40% in processing environments as observed in Table 3. The soaring prevalence of L. monocytogenes recorded for chicken meat and processing environment could be ascribed to the fact that less attention is paid to biosecurity and hygienic measures (Adzitey et al., 2013). The highest prevalence rate of 60% was found in raw chicken from the UK (Pini and Gilbert, 1988), 45% from poultry wing meat in Turkey (Elmali et al., 2015), 20% from raw chicken in Malaysia (Goh et al., 2012), 12.5% from fresh poultry meat in Pakistan (Mahmood et al., 2003), and the lowest contamination rate was 2.50% from frozen chicken meat in Thailand (Kanarat et al., 2011). In a country like Malaysia, most of the live birds are slaughtered and processed at wet markets without authority supervision; because of widespread human handling involved in the processing of chicken, each step of processing is susceptible to cross contamination. In another study by
Table 4: Prevalence (%) of antimicrobial resistance L. monocytogenes among chicken and their processing environment
|
Antimicrobial agent |
Prevalence (%) Sakaridis et al., (2011) |
Prevalence (%) Osaili et al., (2011) |
Prevalence (%) Miranda et al., (2008) |
Prevalence (%) Gómez et al., (2014) |
Prevalence (%) Lyon et al., (2008) |
Overall (%) Prevalence |
|
Amoxicillin |
0.0 |
0.0 |
- |
- |
- |
0.0 |
|
Ampicillin |
0.0 |
- |
- |
- |
- |
0.0 |
|
Cefotaxime |
0.0 |
- |
- |
- |
- |
0.0 |
|
Cephalothin |
0.0 |
- |
- |
- |
- |
0.0 |
|
Chloramphenicol |
0.0 |
0.0 |
3.1 |
0.0 |
- |
3.1 |
|
Ciprofloxacin |
0.0 |
0.0 |
- |
0.0 |
3 |
3.0 |
|
Clindamycin |
83.6 |
- |
- |
35.5 |
0.0 |
59.5 |
|
Enrofloxacin |
0.0 |
0.0 |
- |
- |
- |
0.0 |
|
Erythromycin |
0.0 |
0.0 |
6.3 |
- |
- |
6.3 |
|
Gentamicin |
0.0 |
0.0 |
0.0 |
- |
- |
0.0 |
|
Kanamycin |
0.0 |
- |
- |
- |
- |
0.0 |
|
Nalidixic acid |
100 |
- |
- |
- |
- |
100 |
|
Neomycin |
0.0 |
- |
- |
- |
- |
0.0 |
|
Oxolinic acid |
100 |
- |
- |
- |
- |
0.0 |
|
Oxytetracycline |
9.1 |
- |
- |
- |
- |
9.1 |
|
Penicillin G |
0.0 |
- |
- |
0.0 |
- |
0.0 |
|
Streptomycin |
0.0 |
- |
- |
- |
- |
0.0 |
|
Sulfamethoxazole- Trimethoprim |
0.0 |
0.0 |
15.6 |
- |
0.0 |
15.6 |
|
Tetracycline |
12.7 |
11.8 |
- |
0.5 |
3 |
7.0 |
|
Vancomycin |
0.0 |
- |
0.0 |
- |
- |
0.0 |
|
Tilmicosin |
- |
17.6 |
- |
- |
- |
17.6 |
|
Doxycycline |
- |
0.0 |
18.8 |
- |
- |
18.8 |
|
Oxacillin |
- |
- |
- |
100 |
90 |
95 |
|
Ceptriazone |
- |
- |
- |
- |
59 |
59 |
|
Linezolici |
- |
- |
- |
- |
0.0 |
0.0 |
NB: —, not done.
Johnson et al. (1990), other factors such as breeds of animals, differences in geographical locations, slaughtering processes, handling practices, and storage conditions have greatly influenced the presence of Listeria in chickens.
The prevalence of L. monocytogenes in chicken processing environment is comparatively high as shown in Table 3. It reaches almost 55% in France (Salvat et al., 1995), 40% in Brazil (Loura et al., 2005), and the lowest is 8.9% in Algeria (Bouayad et al., 2015). With the relatively high incidence of L. monocytogenes, chickens and their processing environments remain essential transmission vehicles for these pathogens. Moreover, the formation of biofilm on the chicken carcass-contact surfaces can be a source of cross-contamination when improperly cleaned, thus creating a serious food safety concern (Wilks et al., 2006). L. monocytogenes is capable of adhering to various types of food-contact surfaces that are found in the food processing environments (Di Bonaventura et al., 2008).
The antimicrobial resistance of L. monocytogenes from chicken meat and its related processing environment obtained from several surveys is shown in Table 4. The resistance is wide-ranging according to sample size, type of study, and country involved. Nevertheless, some conclusions were similar to other findings. For instance, no resistance to amoxicillin, enrofloxacin, gentamycin, penicillin G, and vancomycin was observed in this survey. In addition, susceptibility to ampicillin, cefotaxime, cephalothin, kanamycin, neomycin, and streptomycin was 100%. Lower resistance (<10%) was also observed for chloramphenicol, ciprofloxacin, erythromycin, oxytetracycline, and tetracycline. High resistances occurred for sulphamethoxazole-trimethoprim (15.6%), tilmicosin (17.6%), ceftriaxone (59%), clindamycin (59.5%), oxacillin (90%), and nalidixic acid (100%). More significantly, all the examined studies reported a resistance of L. monocytogenes to sulphamethaxol-trimethoprim, chloramphenicol, tetracycline, and ciprofloxacin, thus emphasising the importance of these antibiotics in poultry production industry. Sakaridis et al. (2011) reported that L. monocytogenes isolates from chicken slaughter house displayed high resistance to clindamycin (83.6%) and nalidixic acid (100%). In Malaysia, 15 L. monocytogenes isolates from ducks and their environment were susceptible to the majority of the antibiotics examined, with the exception of 100% resistance to nalidixic acid, 7.0% resistance to tetracycline, and 7.0% resistance to norfloxacin (Adzitey et al., 2013).Thus, L. monocytogenes are steadily more resistant to one or more antibiotics and may signify a possible risk for public health because these antibiotics are commonly used to treat human listeriosis.
CONCLUSION
Relatively high prevalence of Salmonella spp. and L. monocytogenes observed in chickens and their processing environments, as highlighted in this review, may possibly be considered as potential sources of human foodborne illnesses. Strict hygienic practices of slaughter and in chicken processing environment have to be cautiously observed. This review also recognised a high occurrence of antibiotic-resistant Salmonella and Listeria monocytogenes isolated from chicken meat and their related processing environments. The high prevalence of resistance might be attributed to the unrestrained use of antimicrobial drugs as growth promoters, prophylaxis, and therapeutics, with the farmers having unrestricted access to their use. Cautious use of antimicrobials based on correct doses will be essential to safeguard these drugs for clinical usages in humans and animals. There is an urgent need for the establishment of standardised surveillance and monitoring systems for determining the occurrence of resistant foodborne pathogens among foods of animal origin.
ACKNOWLEDGEMENTs
The author acknowledge the following support: Universiti Sains Malaysia for providing USM Global Fellowship to Abatcha Mustapha Goni.
CONFLICT OF INTEREST
There is no conflict of interest in this review to declare.
AUTHORS’ CONTRIBUTION
All the works in this review was performed by Mustapha Goni Abatcha.
REFERENCES