Advances in Animal and Veterinary Sciences
Research Article
Serum Biochemical Investigations on Retained Placenta in Egyptian Buffaloes
Mervat Hassan1, Nani Nasreldin2, Marwa El-Zeftawy3,4*
1Theriogenology Department, Faculty of Veterinary Medicine, The New Valley University, New Valley, Egypt; 2Pathology and Clinical Pathology Department, Faculty of Veterinary Medicine, The New Valley University, New Valley, Egypt; 3Biochemistry Department, Faculty of Veterinary Medicine, The New Valley University, New Valley, Egypt;
4Biological Screening and Preclinical Trial Lab, Biochemistry Department, Faculty of Science, Alexandria University, Alexandria, Egypt.
Abstract | Retained placenta (RP) is one of the reproductive syndromes that occurs in dairy buffaloes where fetal membranes fail to be expelled during 12-hours following parturition. RP cause several economic losses as a result of reduced milk production and infertility resulting prolonged calving interval in affected animals. The purpose of this research was to investigate some proteins alterations at molecular level in relation to metabolic, oxidative, hormonal and immunological changes in Egyptian buffaloes exposed to RP compared to others without RP and non-calved ones. Vaginal examination and rectal palpation were performed to inspect any abnormalities. Blood glucose (BG), lipid and protein profiles, alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea and creatinine levels were estimated by biochemical techniques. Some macro and micro-minerals concentration were also analyzed. The antioxidant defense system was studied via measuring nitric oxide (NO), malonaldehyde (MDA) and reduced glutathione (GSH) concentrations and total antioxidant capacity (TAC) and specific activity of superoxide dismutase (SOD). Further, progesteron (P4) and 17β-estradiol (E2) concentration were explored. Different protein amount percentage was determined using SDS-PAGE electrophoresis. As well as, gene expression of interleukin-1beta (IL-1β) and interleukin-6 (IL-6) was evaluated. In RP group, the obtained data exhibited reduction of BG and lipid profile with exception of very low density lipoprotein. Likewise, alterations of protein were noticed including elevation of globulin and diminishing of albumin. Pro-oxidant parameters (MDA and NO) were elevated; whereas, antioxidant factors (TAC, GSH and SOD) were declined. Moreover, urea concentration, ALT and AST activities were significantly increased, while there wasn’t change in creatinine level. At hormonal level, a reduction of E2 and elevation of P4 were noticed and the gene expression of both IL-1β and IL-6 was declined. From the obtained results we can conclude that RP accompanied by low immunity and oxidative stress which directly affect metabolic and hormonal conditions of the animals and it influences proteins amount in the body which is responsible for the body defense.
Keywords | Retained placenta, Egyptian buffaloes, Oxidative stress, Inflammation, Cytokines.
Received | September 14, 2019; Accepted | December 23, 2019; Published | January 03, 2020
*Correspondence | Marwa El-Zeftawy, Biochemistry Department, Faculty of Veterinary Medicine, The New Valley University, New Valley, Egypt.; Email: marwa@vetnv.au.edu.eg; marwa_3_1983@yahoo.com
Citation | Hassan M, Nasreldin N, El-Zeftawy M (2020). Serum biochemical investigations on retained placenta in Egyptian buffaloes. Adv. Anim. Vet. Sci. 8(1): 67-76.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.1.67.76
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 El-Zeftawy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Buffaloes constitute a highly economic importance as they participate in meat and milk production in several developing countries including Egypt. Reproductive disorders such as delayed puberty onset, poor estrus cycle, dystocia, metritis and retained placenta (RP) have a direct negative impact on the reproductive efficiency in buffaloes (Fareed et al., 2017).
Placenta is a temporary endocrine gland where part of it develops from maternal uterine tissues while the other part develops from blastocyst which forms the fetus. In normal condition, placenta is expelled within 12-hours after delivery (Tucho and Ahmed, 2017). The main role of placenta is focused on nutrients and oxygen transfer from dam to the embryo, in addition it secretes many hormones to support pregnancy and fetal growth (Burton and Fowden, 2015).
RP is a pathological reproductive condition results due to failure of the expulsion of all or part of the placenta or fetal membranes after delivery through 12 hours (Tucho and Ahmed, 2017). The fundamental causes of RP fluctuated from twine birth, nutritional deficiency, low immunity and environmental and hormonal causes (Pontes et al., 2015). RP produces several harmful effects inside reproductive organs of animals because it allows growth of microorganisms inside the uterus causing uterine inflammation (Kimura et al., 2002). In addition, fever, weight loss and reduction of milk yield are common adverse effects of RP syndrome and in severe cases animal death may occur due to hemorrhage (Tagesu, 2018). Other risk factors associated with RP include delayed uterine involution, ovarian cystic degeneration, chronic endometritis, pyometra and fertility reduction (Ganaie et al., 2018).
The idea of current work came from the increased rates of multiple gynecological problems in Egyptian farms which may lead to livestock economic losses in the future and one of those problems is RP syndrome. Hence studying some metabolic pathways of RP may be a helpful tool to treat the condition before its onset. So, this study was directed to evaluate the adverse effect of delayed placental expulsion (RP) 24-hours post parturition in Egyptian buffaloes via the investigation of some biochemical and immunological parameters in comparison with non-retained (NRP) placenta group and heifers (HEF) that did not yet give birth to a calf.
Material and Methods
Reagents and Chemicals
Kits of total protein (TP), blood glucose (BG), total cholesterol (TC), triacylglycerol (TG) and high density lipoprotein-cholesterol (HDL-c) were got from Diamond (Egypt), photometric albumin, urea and creatinine and kinetic alanine aminotransferase (ALT) and aspartate aminotransferase (AST) kits were purchased from Human Co. (Germany). Total antioxidant capacity (TAC) kit was bought from Biodiagnostic Co. (Egypt). As well as kits of calcium (Ca2+), magnesium (Mg2+), copper (Cu2+), zinc (Zn2+) and total iron (TF) were obtained from Spectrum Co. (Germany).
Protein and deoxy ribonucleic acid (DNA) ladder and complementary deoxy ribonucleic acid (cDNA) kit were purchased from Biovision (USA). Serum ribonucleic acid (RNA) purification and Maxime reverse transcription (RT) Premix Kits were got from NORGEN (Canada) and INtRon Biotechnology (INC). Enzyme linked immuno assay (ELISA) kits of progesterone (P4) and 17β-estradiol (E2) were obtained from DiaMetra (Italy).
Acrylamide, sodium dodecyl sulfate (SDS), ß-mercaptoethanol, thiobarbituric acid, trichloroacetic acid, sodium nitroprusside, sulfanilamide, N-naphthyl-ethylenediamine dihydrochloride, 5′-5′-dithio bisnitrobenzoic acid, reduced glutathione (GSH) standard, Tris HCl, superoxide dismutase (SOD) standard, pyrogallol, agrose, boric acid, ethidium bromide, bromophenol blue and other organic solvents were purchased from Sigma-Aldrich Chemical Co. (USA). Diethylene triaminopenta acetic acid and 5-sulfosalicylic acid dehydrate were obtained from ACROS CO. (USA, Belgium) and Nour Resh,shark Co. (Egypt) respectively.
Study Design, Gynecological Examination and Blood Samples Collection
The study was conducted on 3 dairy farms at Assiut province, Egypt (Arab orders center Abnob, village Bani Mor and village bathroom of Abnob center) from 2016 till 2017. The climatic temperature of the farm ranged between 12oC in winter and 45oC in summer months and relative humidity 41-85%. Buffaloes were fed ad-libitum seasonal green food and concentrate mixture. The study was conducted on non-parturated buffaloes which named HEF and others subjected to third time of parturition and there wasn’t any interference during the pregnancy or parturition. Gynecological inspection via rectal palpation and vaginal examination post parturition was performed for all calved buffaloes and time of placenta expulsion was observed and it was reported that some buffaloes weren’t expelled their placenta within 12 hour. Hence, our study was performed on those buffaloes. On day 2 following parturition blood specimens were collected from jugular vein, and all efforts were done to decrease the distress. The samples were obtained from 287 calved buffaloes throughout two years where only 40 samples were selected for the current study depending on time of placental expulsion. Other 20 samples were collected from non-calved buffaloes (HEF). So, the samples were divided into three groups, group I HEF group (n=20), group II buffaloes expelled their placenta within 12 hours after parturition (NRP group) (n=20) and group III buffaloes didn’t expel their placenta within 12 hours and were diagnosed with RP (RP group) (n=20).
Biochemical Study
Fasting serum BG was determined on the base of glucose-oxidase peroxidase method. Serum TC, TG and HDL-c was determined using commercial kits. However low density lipoprotein cholesterol (LDL-c) and very low density lipoprotein cholesterol (VLDL-c) levels were calculated according to previous formula of (Fawwad et al.,
Table 1: Primer sequence and PCR product length
| Gene | Primer sequence | Gene bank accession No. | Product length | |
| G3PDH | F |
5′-GCCAGTAGAAGCAGGGATGA-3′ |
NM_001190390.1 | 423 bp |
| R |
5′-AAGGCCATCACCATCTTCCA-3′ |
|||
|
IL-1β |
F |
5′-TGGCACTCTAACCCGGAAAT-3′ |
NM_174093.1 | 449 bp |
| R |
5′-AAAGCCATACCCAGGGAGTC-3′ |
|||
| IL-6 | F |
5′-GTCAGTGTTTGTGGCTGGAG-3′ |
NM_173923.2 | 444 bp |
| R |
5′-ACTTCTGCTTTCCCTACCCC-3′ |
|||
2016; Srilatha et al., 2017) respectively. Also, kinetic method was performed to measure ALT and AST activity in serum samples according to instruction of kits. However, colorimetric procedure was used to assay both urea and creatinine concentrations in all examined samples. TP was analyzed according to previous method recorded by Witt and Trendelenburg (1982). Serum albumin was assayed according to manufacture of kit’s instructions and globulin concentration and albumin/globulin (A/G) ratio were calculated (Samanta et al., 2016).
Biochemical estimation of some macro and micro-minerals was also performed in our work. Analysis of Zn2+ was done dependent on Johnsen and Eliasson (1987) method. Also, Ca2+ level in serum samples was determined by colorimetric quantitative method according to kits instruction. In addition, the colorimetric determination of TF, Mg2+ and Cu2+ was carried out according to Williams et al. (1977), Farrell (1984) and Young (2001) respectively.
Moreover, serum lipid peroxidation (LPO) was estimated by the reaction of malonyldialdehyde (MDA) as a major secondary product of LPO with thiobarbituric acid to produce thiobarbituric acid reactive substances (Łukaszewicz-Hussain et al., 2007). Nitric oxide (NO) level was also measured using Chavda and Udhnawala (2019) technique with slight modification.
Serum TAC was performed by colorimetric enzymatic reaction method (Koracevic et al., 2001). GSH content was determined as described previously (Giustarini et al., 2017). Specific activity of serum SOD was also assessed depending on the ability of SOD to prevent auto-oxidation of pyrogallol (Marklund and Marklund, 1974).
Hormonal analysis of serum P4 and E2 were also done individually via their specific ELISA kits according to guidelines of their instructions.
Molecular Study
Molecular study of serum IL-1β and IL-6 was carried out and G3PDH was used as an internal control gene which is termed also housekeeping gene where it was used as an internal standard and it isn’t affected by any experimental disorders. First total RNA was isolated from serum samples by spin column method using commercial kits according to manufacture of instructions. After that, isolated RNA was reverse transcribed to cDNA according to kits guidelines where 1 μg of template RNA and RNase free H2O were added into the Maxime RT pre mix to a total volume of 20 µl. After that the mixtures were let stand at RT tubes for 2 min., then the mixtures were dissolved well by pipetting and cDNA synthesis reaction was performed by using polymerase chain reaction (PCR) machine according to the following conditions: step of DNA synthesis at 45oC for 60 min. and RTase inactivation step at 95oC for 5 min.
For gene expression normal PCR was performed, the nucleotide sequence of gene-specific primers were constructed using NCBI data base (Table 1). PCR program was adjusted for each primer pair using PCR thermal cycle. The reaction mixture was consisted from 12.5 µl of 2X Taq master mix, 1 µl of diluted forward primer, 1 µl of diluted reverse primer, 5.5 µl of RNase free H2O and 5 µl of cDNA which was used as template to amplify G3PDH, IL-1β and IL-6. The mixtures were mixed well using vortex and entered in PCR machine where PCR thermal cycle was carried and it was started by initial denaturation at 94oC for 2 min. followed by 35 cycles of PCR, each consisted of 2 sec. denaturation at 94oC, 30 sec. annealing at 64oC for G3PDH, 64.5oC for IL-1β and 64.1oC for IL-6 and final cycle was 30 sec. extension at 72oC.
Then gel electrophoresis was performed for PCR product and DNA marker used as a positive indicator using 1.5% agrose gel in Horizontal Electrophoresis unit and illuminated with 30 nm U.V Radiator and photographic record was made. Optical density of bands was calculated by GelQuantNET software program. Then relative gene expression was calculated according to the following equation:
Relative gene expression =
Additionally sodium dodecyl sulfate poly acrylamide gel electrophoresis (SDS-PAGE) of serum samples was done to identify if there was any alteration in protein profile in all studied groups. It was performed after determination of TP concentration according to Green and Sambrook (2012) where 27μg of protein sample was subjected to electrophoresis in presence of 30% acrylamide, detergent SDS and reducing agent ß-mercaptoethanol using Eco-Mini Vertical Electrophoresis unit. Protein bands were visualized then illuminated by gel documentation system and molecular weight (Mwt) of visualized protein bands was determined by comparing them with Mwt of protein ladder. The percentage of protein amount in each sample was calculated using Documentation Program Gel Pro Analyzer.
Statistical Analysis
Results were computed and statistically analyzed by means of the program SPSS (Version 16, one way ANOVA-test). Results are presented as mean ± SE and the p-value < 0.05 was reflected statistical significant.
Results
The incidence of RP was recorded as 19.04% in studied group of calved buffaloes used for the current work. Gynecological examination of RP group showed presence of persistent corpus luteum (CL) on ovaries and some buffaloes were suffered from endometritis with bloody purulent offensive vaginal discharge after 2 days postpartum. Also rectal palpation of HEF group revealed absence of CL which indicates the studied HEF was cyclic in follicular phase.
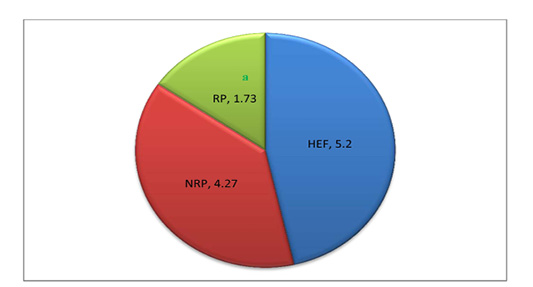
Gene expression of IL-1β and IL-6 against glycerol-3-phosphate dehydrogenase (G3PDH) was revealed in Table 2 and Figure 1. where they decreased by 80.9% and 9.6% respectively in RP comparing to NRP. Comparing by HEF, NRP showed highly significant elevation of both IL-1β and IL-6 (P<0.001) while P-value of RP in case of IL-1β was <0.001 and <0.01 in case of IL-6. Also, serum TP level was decreased by 13.0% and albumin was also declined by 50.2% in RP comparing to NRP. However, serum globulin was increased by 30.1% in RP. Also, reduction of calculated A/G ratio was observed (P<0.05) in case of RP (Figure 2). Significant decline of TP in NRP and RP (8.32 ± 0.26 and 7.24 ± 0.16, respectively) was noticed compared to HEF (10.32 ± 0.52). In group of RP only there was significant decline of albumin compared to HEF (P<0.001). However globulin was increased in RP and NRP and A/G ratio was significant declined compared to HEF.
SDS-PAGE was used to differentiate between HEF, NRP and RP groups according to their protein extract content and the results are recorded in Figure 3. where protein ladder was separated into several bands (Mwt from 6.5 to 270 KDa). HEF group was separated into nearly 18 bands with
Table 2: Gene expression of serum IL-1β and IL-6 against G3PDH in Egyptian buffaloes before and after calving in presence and absence of RP
| Groups |
IL-1β/G3PDH |
IL-6/G3PDH |
| HEF | 0.05 ± 0.01 | 0.31 ± 0.03 |
| NRP |
0.47 ± 0.02a |
0.73 ± 0.01a |
| RP |
0.09 ± 0.01b |
0.66 ± 0.01a |
Values represent mean ± SE. SPSS version 16 (one way ANOVA test). Significance: aP < 0.001, bP < 0.01, cP < 0.05 comparing to HEF.
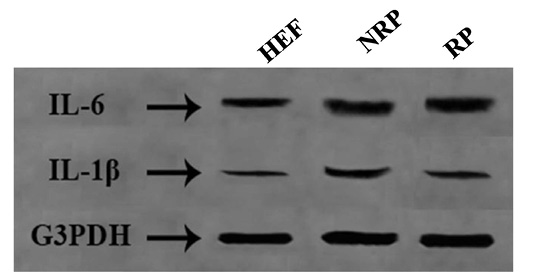
Figure 1: Agrose gel electrophoresis of IL-1β (449 bp) and IL-6 (444 bp) against G3PDH (423 bp) as a house keeping gene.
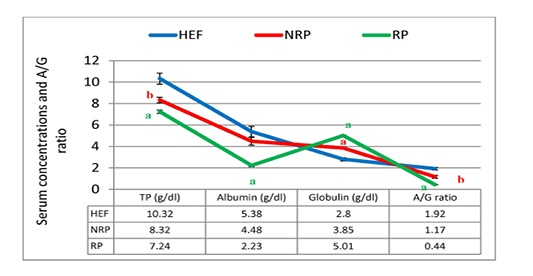
Figure 2: TP, albumin, globulin and A/G ratio of serum Egyptian buffaloes before and after calving in presence and absence of RP. Values are expressed as mean ± SE (n=20). Significance: aP < 0.001, bP < 0.01, cP < 0.05 comparing to HEF.
varied Mwt from (7 to 130 KDa). NRP group was separated into nearly 16 bands with different Mwt from (7 to 165 KDa). RP group was separated into nearly 14 bands with different Mwt from (7 to 173 KDa). Several differences in serum protein composition were obvious between the three groups. From those variations increase the levels of a 58- and 57-KDa in the serum of HEF and RP as compared to NRP and those proteins demonstrated to be collagen. Another difference was decrease the levels of a 25- and 17-KDa in case of HEF and RP than NRP group and those proteins were revealed to be IL-6 and IL-1β respectively, however the decline was more obvious in HEF. Further presence of a 60-KDa protein in RP that was absent or in low amounts in NRP serum and this protein is shown to be P4. However E2 protein was appeared in a huge quantity at a 70-KDa in HEF and NRP group than RP. Also, it was reported decline of 72-KDa protein in HEF and RP than NRP and this protein recognized to be prostaglandin (PG).
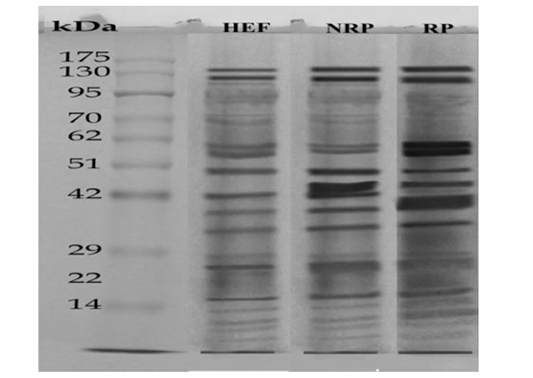
Figure 3: SDS-PAGE of serum (27μg protein) Egyptian buffaloes before and after calving in presence and absence of RP. The separation was done on discontinuous system using 4% stacking gel and 12% separating gel then, fixing, staining and de-staining of obtained gel. Lan (1) represented HEF group, Lan (2) is NRP group and Lan (3) is RP group.
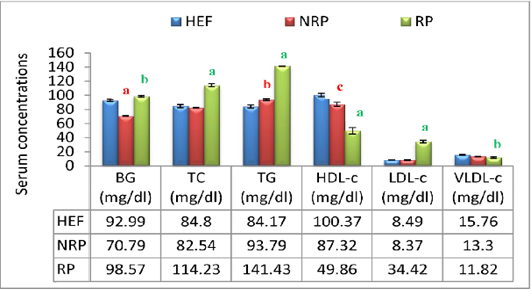
Additionally buffaloes have RP exhibited an increasing affinity in BG level (P<0.001) within 2 days after parturition compared to NRP and HEF. At lipid profile level it was noticed in RP group elevation of serum TC and TG by 27.7% and 33.7% respectively, while HDL-c was reduced by 42.9% comparing to NRP. Further, serum LDL-c in relation to RP revealed significant increase (P<0.01) however; VLDL-c hasn’t any significant variation comparing to NRP. By comparing NRP by HEF at lipid profiles it was reported significant elevation of TG (P<0.01) and fall of HDL-c (P<0.05). Additionally RP showed significant increase of TC, TG and LDL-c (P<0.001) decrease of HDL-c and VLDL-c (P<0.001 and <0.001, respectively) when compared to HEF (Figure 4).
In Figure 5 and 6 serum MDA, NO, SOD, GSH and TAC were shown. Serum MDA and NO were found to be significantly elevated (~3.8 and 1.3-folds, respectively) in buffaloes with RP than NRP. While SOD specific activity, GSH concentration and TAC were decreased 59.5%, 57.1% and 75.4% respectively. Also, NRP showed significant increase of NO (0.39 ± 0.04) compared to HEF (0.19 ± 0.02) and reduction of GSH and TAC (P< 0.05 and < 0.001, respectively). Moreover, RP showed significant rise of MDA and NO and dimension of SOD, GSH and TAC (P< 0.001) in comparison to HEF.
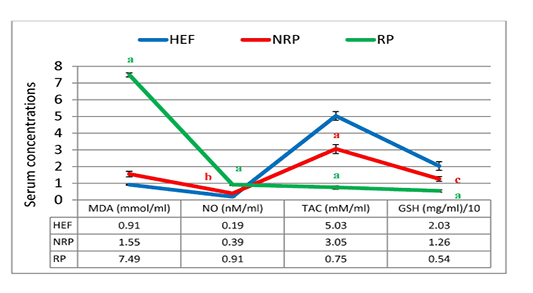
Figure 5: MDA, NO, TAC and GSH of serum Egyptian buffaloes before and after calving in presence and absence of RP. Values are expressed as mean ± SE (n=20). Significance: aP < 0.001, bP < 0.01, cP < 0.05 comparing to HEF.
At hormonal level it was observed that serum E2 was diminished by 61.1% in RP however P4 was elevated by 35.6% in RP compared to NRP group. Further, E2 level was significant decline in NRP and RP (94.30 ± 3.21 and 36.73 ± 5.50, respectively) compared to HEF (115.86 ± 2.18). However P4 level was increased in both NRP and RP (6.73 ± 0.38 and 9.13 ± 0.15, respectively) comparing with HEF (4.69 ± 0.21) (Table 3).
Table 3: Serum concentrations of E2 (pg/ml) and P4 (ng/ml) of Egyptian buffaloes before and after calving in presence and absence of RP
| Groups |
E2 (pg/ml) |
P4 (ng/ml) |
| HEF | 115.86 ± 2.18 | 4.69 ± 0.21 |
| NRP |
94.30 ± 3.21b |
6.73 ± 0.38a |
| RP |
36.73 ± 5.50a |
9.13 ± 0.15a |
Values represent mean ± SE. SPSS version 16 (one way ANOVA test). Significance: aP < 0.001, bP < 0.01, cP < 0.05 comparing to HEF.
Table 4: Serum activities of ALT and AST (I/U) and levels of urea and creatinine (mg/dl) in Egyptian buffaloes before and after calving in presence and absence of RP
| Groups | ALT (I/U) | AST (I/U) | Urea (mg/dl) | Creatinine (mg/dl) |
| HEF | 27.57 ± 0.37 | 25.96 ± 0.55 | 20.69 ± 0.95 | 0.93 ± 0.04 |
| NRP | 27.77 ± 0.94 | 26.13 ± 0.86 | 24.33 ± 0.67 | 1.07 ± 0.09 |
| RP |
46.53 ± 1.82a |
38.17 ± 2.55b |
37.0 ± 1.73a |
0.9 ± 0.02 |
Values represent mean ± SE. SPSS version 16 (one way ANOVA test). Significance: aP < 0.001, bP < 0.01, cP < 0.05 comparing to HEF.
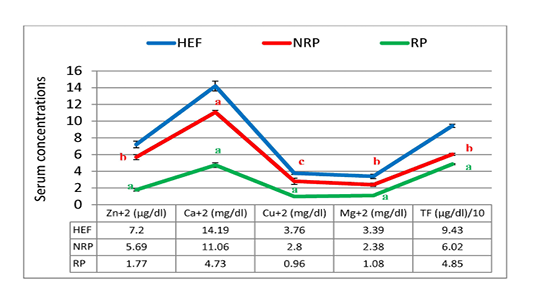
Figure 7: Zn+2, Ca+2, CU+2, Mg+2 and TF of serum Egyptian buffaloes before and after calving in presence and absence of RP. Values are expressed as mean ± SE (n=20). Significance: aP < 0.001, bP < 0.01, cP < 0.05 comparing to HEF.
Moreover the mean of studied Ca2+ and Mg2+ values showed reduction trend by 57.23% and 54.62% respectively in case of RP compared to NRP. Also, essential trace minerals in RP revealed decline in Zn2+, Cu2+ and TF by 68.9%, 65.7% and 19.4% respectively. Additionally all studied minerals exhibited significant reduction in both NRP and RP groups compared to HEF group (Figure 7). Also, serum ALT and AST activities and urea concentration was elevated by 67.6%, 46.1% and 52.1% respectively in case of RP compared to NRP and also significant elevation was reported when compared to HEF (P< 0.001 and < 0.01). Conversely, there was no significant difference in serum creatinine level between RP and NRP and HEF groups (P>0.05) (Table 4).
Discussion
Reproductive problems in buffaloes related to parturition take more attention nowadays due to their side effects on the production and fertility with subsequent economic loss. The normal sequence of parturition is influenced by number of endocrine and paracrine factors. It begins with gradual decrease of placental P4 concentration and increased production of E2 hormone where E2 has a vital role in the growth of placentomes and PG release (Attupuram et al., 2016). The role of albumin during normal parturition occupies huge significance as it is a source of endogenous amino acids which are required postpartum (Bhat et al., 2018).
Parturition is provoked by inflammation that leads to uterine contractions with subsequent ejection of the newborn and the fetal membranes. Several pro-inflammatory mediators, containing cytokines, are released into the feto-maternal interface for the preparation to parturition and during it. This starts, in humans and cows, from recognition of the fetal antigens by maternal immune system in such a way similar to a graft rejection like reaction (Jaworska and Janowski, 2019). Pro-inflammatory cytokines, IL-1β and IL-6, participate in the events of parturition by activation of immune cells and they are linked to PG signaling pathway (Keelan et al., 2003). IL-1β, IL-6 and, tumor necrosis factor alpha (TNF-α) are biomarkers which their expression significantly elevates in placental tissues throughout parturition in human and cows. Also, both IL-1β and TNF-α enhance PG production and they are able to activate the nuclear factor kappa-B signaling pathway, which further stimulates myometrial contractions. In addition, the inflammatory process must be properly tuned to ensure that it will not exert any harmful effect along the course of parturition and, subsequently, the mother and the fetus. An altered inflammatory response in cows is presumably one of the causes that lead to RP (Jaworska and Janowski, 2019). Our data revealed significant decrease in IL-1β gene expression, this result is in the same aspect with Islam et al. (2013) who recorded significant reduction in the serum level of IL-1β fifteen days before parturition and continued to the fifteenth day post-delivery which indicates disturbed immune response with subsequent RP. Also, our results match with (Shimizu et al., 2018) who found that IL-1β gene expression was significantly lower in peripheral blood mononuclear cells at 4 weeks postpartum in RP dairy cows than control ones. IL-6 has a role in the normal physiological processes of parturition as it is expressed in the female reproductive tract and gestational tissues and also it regulates the growth of placenta and helps in embryo implantation (Gomez-Lopez et al., 2016). It was reported that IL-6 affects low P4 level and activates genes of uterus which are responsible for normal delivery (Robertson et al., 2010). Moreover, IL-6 stimulates the adaptive immune response which may take place throughout parturition (Jaworska and Janowski, 2019). In this work, IL-6 gene expression was significantly decreased in RP in comparison with NRP, this result is in the same line with (Ishikawa et al., 2004) who recorded low level of the peripheral blood IL-6 before parturition which associated with defect in placental function that contribute to RP. They suggested that the cause of RP was due to abnormality of the shift in the immune response during delivery.
Moreover, RP is associated with imbalances in E2 and P4 levels. High P4 and low E2 levels were occurred due to failure of the placenta to produce sufficient amounts of cytochrome P450 aromatase enzyme which has a role in catalyzing the rate limiting step in E2 biosynthesis (Attupuram et al., 2016). Further, lower levels of IL-6 in RP syndrome affected buffaloes may lead to reduction in the expression of Cyp19 gene which encodes cytochrome P450 aromatase enzyme so; inadequate E2 production will be happened leading to the formation of undeveloped placentomes which aren’t expelled out naturally causing RP (Ghai et al., 2012).
Also, prostaglandins have a key role in normal delivery through uterine contraction activation and they help in the release of the placenta (Grillo-Ardila et al., 2018). Production of PG is usually connected to E2 so reduction of PG level in RP can be a consequence of E2 deficiency (Yasuhara et al., 2019). Further prostaglandin-F-2-alpha (PG-F2α) is responsible for CL lysis hence it is suspected that low level of PG-F2α is one of contributing factors for enhancement RP syndrome (Parmar et al., 2016). Our results at protein level exhibited low PG in RP which parallel to those Wischral et al. (2001) who stated that PG-F2α metabolites decline in cows failed to release their fetal membranes.
As a consequence of tissue destruction and inflammation during the period of RP in attempts to eject the placenta, the production of protein in liver is partly altered towards increased synthesis of positive acute phase proteins and reduction of albumin which is a negative acute phase protein and elevation of globulin, hence diminishing of A/G will be occurred (Saleh et al., 2008). In addition, the current results revealed a raise in the level of the serum collagen in RP group which attributed to its failing to perform the normal function as it wasn’t consumed in placenta removing process (Beagley et al., 2010). Normally, during parturition caruncle cotyledon collagen had been broken under the effect of , matrix metalloproteinase type-2 (MMP-2) to facilitate separation of the placenta (Hiebel et al., 2019). Our data came in accordance with Dilly et al. (2011) who noticed elevation of MMP-2 in animals has RP than others so direct relationship between MMP-2 and collagen level.
Moreover, it was recognized that buffaloes which released their placenta within 12 h postpartum showed decline in glucose level due to utilization of glucose required for lactose synthesis (Cui et al., 2019). However, the current data observed hyperglycemia and hyperlipidemia in buffaloes with RP which is expected due to insulin resistance that linked with RP while the concentration of insulin wasn’t measured in our study. Another hypothesis of hyperglycemia may be attributed to elevation of cortisol as a consequence of stress factor produced from RP (Cheung et al., 2019). Hypocalcemia which observed in RP may be another factor of hyperglycemia because the decline of Ca2+ level prevents insulin production (Chen et al., 2016). Also, severe hyperlipidemia in RP leads to accumulation of lipids inside the hepatocytes and subsequently increase in the activity of the liver enzymes and liver injury (Joksimovic-Todorovic and Davidovic, 2013).
In general parturition is considered as one of stress factors on animals due to changes in O2 use and pressure and it is associated with reactive oxygen species (ROS) production. RP constitutes more stress on the animals which become more susceptible to immune suppression (Kankofer et al., 2010). The imbalance in ROS production is one of the predisposing factors which cause improper release of placenta. Hence study of antioxidant defense system such as TAC, SOD and GSH was very crucial matter during current work. The present work showed reduction of SOD activity, TAC and GSH level in RP comparing to NRP which attributed to multi factors including the reduced production of E2 and PG-F2α and accumulation of arachidonic and linoleic acids in the placental tissue (Abdisa, 2018). Concerning to SOD decline, it is expected due to shortage of some micro-minerals concentration such as Cu2+ and Zn2+. From biochemical theme Cu2+ and Zn2+ are involved in the function of SOD enzyme, so reduction of those minerals indirectly proportional with SOD activity. Further, lower serum TAC in RP is attributed to disturbances in anti-oxidative/oxidative balance and is confirmed by reduction of SOD specific activity. Current findings is confirmed by previous data of Kankofer et al. (2005) who noticed an elevation of TAC in placental tissue of cows affected with RP as compared to those without RP which indicates a transfer of TAC from blood to placental tissue.
Moreover, our results exhibited that RP is associated with elevation of MDA and NO concentration which come in line with preceding studies (Jovanovic et al., 2013; Islam and Kumar, 2015). MDA is an indicator of lipids peroxidation which associated with presence of poisonous metabolites and destruction of free fatty acids and phospholipids (Erisir et al., 2006). We assumed that high level of MDA was predicted as a result of metabolic and endocrine changes related to RP (Yildiz et al., 2011). While, elevation of NO may be related to the imbalance of P4 concentration leading to NO metabolites production which are the main source of elevated NO level where both NO and P4 play a significant role in preserving placental perfusion during the gestational period by inducing smooth muscle relaxation (Chwalisz and Garfield, 1998).
As a consequence of alteration of anti-oxidative defense system during RP, various degrees of renal and hepatic tissue injuries will be occur which may be attributed to the impairment of the mitochondrial function during the oxidative stress (Cahova et al., 2015).
In this study the effect of RP on some minerals was displayed where the imbalance was clearly observed and directly effect on several metabolic pathways inside buffaloes’ body. Gynecological inspection showed reduction of uterine muscle contractility in case of RP which attributed to shortage of Ca2+ (Talukdar et al., 2016). From biochemical background hypocalcemia is usually associated with hypomagnesemia which cause hyperexcitability of uterine muscle. Data in the current work are like to previous study in cows (Qu et al., 2014).
Also, it was reported that buffaloes with RP significantly deficient in Zn2+ which has important role in preserving the uterus following parturition as it helps in healing process and immune system during convalescent stage (Bicalho et al., 2014). In normal physiological conditions most of maternal iron is transferred to the fetus during last trimester of gestation so the reduction of TF in serum of dam is considered normal (Cao and O’brien, 2013). In the light of our findings TF in case of RP was lower than NRP which attributed to Cu2+ reductions as it required for biosynthesis of hemoglobin.
All our obtained results were confirmed by rectal and vaginal examination which revealed increased the size of uterus with presence of bloody and purulent vaginal discharge with offensive odor in buffaloes with RP and this was in harmony with previous work (Khan et al., 2015).
Conclusion
From this study, it was concluded that the lower immunity (reduction in IL-6 and IL-1β) during parturition in Egyptian buffaloes may be the predisposing cause of retained placenta. Buffaloes with retained placenta were predictable to have low level of antioxidant system and high level of pro-oxidants compared to buffaloes without retained placenta. Based on our data in protein profile and minerals concentration in case of heifers, buffaloes with and without retained placenta, we conclude that improvement of nutrition during the pregnancy may serve as a tool for decreasing retained placenta occurrence. Furthermore, to understand the mechanism of low immunity and oxidative stress in relation to retained placenta we need to broaden the spectrum of study so we recommend further studies in such aspect.
Acknowledgments
The authors thank Biological Screening and Preclinical Trial Lab, Biochemistry Department, Faculty of Science, Alexandria University, Alexandria, Egypt for financial support.
Conflict of interest
The authors haven’t conflict of interest to declare.
authors contribution
Mervat Hassan prepared the idea of the manuscript, blood sampling and gynecological examination. Nani Nasreldin analyzed some biochemical parameters and wrote the discussion part of the pro-inflammatory cytokine (IL-1β and IL-6) in the original manuscript draft. Marwa El-Zeftawy analyzed some biochemical parameters and all molecular parameters, she did statistical analysis of the results and wrote the original manuscript draft. Also she was the corresponding author who responded the journal requests during reviewing and editing. All authors are contributed in revising the final draft of the manuscript.
References