Journal of Animal Health and Production
Research Article
Effects of Aspirating Long Needle Rumenocentesis on Haematology and Some Physiological Parameters of Yankasa Sheep in Maiduguri
Abubakar Mshelia Saidu1*, Jummai Ilebaye Abbah1, Usman Mohammed Kolo2, Dauda Laku1, Musa Kalim Adam1
1Department of Veterinary Surgery and Radiology, University of Maiduguri, Borno State, Nigeria; 2Department of Animal Science, University of Maiduguri, Maiduguri, Borno State, Nigeria.
Abstract | Oral probe method of rumen liquor collection affects volatile fatty acid (VFA) concentration, pH and total acidity of the liquor while a caudo-ventral ruminal sac approach with 14-18 gauge needles have been associated with leakage at ventral puncture sites and blockage of needles with the ruminal contents during collection. Aspirating long needle rumenocentesis was employed to obtain rumen liquor for rumen liquor pH evaluation and protozoa motility test as well as expounded the influence of long needle rumenocentesis on haematology and some physiological parameters of Yankasa Sheep in Maiduguri, a semi- arid region of Nigeria. The significant increase in packed cell volume (PCV) (p<0.05) at days 1 and 2, 32.7±1.24%, and 32.7±1.04% respectively, compared to the baseline value (28.9±0.38%). However, these values were within normal species reference range (27-45%). The significant difference (p<0.05) in white blood cell (WBC) at days 1 and 4 (11.20±0.43x103μl and 10.9±0.37x103μl) respectively post rumenocentesis was recorded and compared to the baseline (9.61±0.59x103μl). The significant difference (p<0.05) in eosinophil count was observed from day 1-7 compared to the baseline value. Significant difference not recorded in physiologic parameters (p>0.05) including heart rate, respiration rate and rectal temperature indicates absence of systemic problems. Therefore, aspirating long needle rumenocentesis is a safe and reliable tool for rumen fluid collection in Yankasa breed of Sheep.
Keywords | Rumenocentesis, Ovine, Yankasa Sheep, Aspirating long needle, Haematology
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | February 21, 2017; Accepted | April 21, 2017; Published | April 26, 2017
*Correspondence | Abubakar Mshelia Saidu, Department of Veterinary Surgery and Radiology, University of Maiduguri, Borno State, Nigeria; Email: [email protected]
Citation | Saidu AM, Abbah JI, Kolo UM, Laku D, Adam MK (2017). Effects of Aspirating Long Needle Rumenocentesis on Haematology and Some Physiological Parameters of Yankasa Sheep in Maiduguri. J. Anim. Health Prod. 5(2): 58-63.
DOI | http://dx.doi.org/10.17582/journal.jahp/2017/5.2.58.63
ISSN | 2308-2801
Copyright © 2017 Saidu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Sheep are important component of the rural economy particularly in the tropics or arid, semi-arid and mountainous area of the country Nigeria in that it provides income to the shepherds through sales of wool and animal. They are important in agriculture on account of their unique ability to adapt and maintain themselves under harsh environment (Hassan et al., 2015). Four breeds of Sheep exist in Nigeria, the West African dwarf, Yankasa, Uda and Balami (Simmons et al., 2001). Yankasa is the most numerous breed of Sheep in Nigeria and has the widest distribution, being found throughout the sub-humid and semi-arid zones (Salako, 2013).
Rumenocentesis, the collection of rumen fluid or liquor by percutaneous invasion and aspiration is a diagnostic technique that essentially involves inserting a needle or it likes into the rumen and aspirating its content usually rumen liquor (Bambi et al., 2011), for microbial count, protozoa motility test and determination of rumen pH (Kleen et al., 2004). Rumen fluid can also be obtained through oral or nasal intubation, contrast surgery (Bambi et al., 2011). Rumenocentesis is a technique commonly employed for feed analysis by ruminant feed nutritionist for sampling the rumen content for nutritional analysis of fed diets to ruminants. Rumenocentesis has been recommended for early diagnosis of rumen acidosis which is based on the pH of the rumen fluid as an alternative to oro-ruminal collection using a stomach tube (Tajik et al., 2011). Sheep owners see the need to fatten sheep in order to attract buyer especially during Muslim festive (Id-el Kabir) periods in Maiduguri, as such, diets largely grains rich in carbohydrate are fed to the Sheep. This may predispose ruminants to lactic acidosis which is a common rumen disorder of ruminants and worst in ovine species. These rumen disorders are responsible for huge economic losses in herd industry due to decrease in production and increase in expenditure towards treatment. Skin reaction to the rumenocentesis site was different between fresh and mild lactation cows and primiparious and multiparous cows, therefore some factor such as immune system condition and the level of the cow resistance during rumenocentesis may affect the occurrence of rumenocentesis complication following rumenocentesis using an 18 gauge (18G), 120mm long stainless needle (Tajik et al., 2011). According to Duffield (2004) and Hofírek and Haas (2001), samples collected using oro-ruminal method has high pH values and bicarbonate concentration. This is due to contamination with saliva, and further recommending rumenocentesis as a more appropriate technique for rumen fluid collection since the fluid is collected directly from the rumen with more accurate rumen pH and bicarbonate values (Duffield et al., 2004; Hofírek and Haas, 2001). Several studies have been done in the past to determine the effectiveness and dangers associated with rumenocentesis (Jos, 2011). Although studies on ovine rumenocentesis are very rare, the clinical and pathological consequences of rumenocentesis were evaluated closely in bovine species that showed cutaneous inflammation on the puncture sites with inflammation reading 5cm in diameter in some cows (Strabel et al., 2007). The severe complications encountered with this technique do not legit rumenocentesis as a routine procedure for collection of rumen fluid (Strabel et al., 2007). Rumenocentesis when carried out meticulously will result in no complication in the contrary (Morgante et al., 2007). Several nutritional studies of ruminants are conducted in Maiduguri but there is paucity of information on a detailed rumenocentesis procedure in Sheep unlike the bovine species.
The red blood cell and its indices following rumenocentesis have not been significantly affected in the bovine species, except the white blood cell indices that showed increase as response to stress from surgery and possible inflammation of the puncture sites (Enemark et al., 2004). A marked leucocytosis with neutrophilia will be noted due to stress following rumenocentesis. Clinical or physiologic parameters usually evaluated in animals are body temperature, pulse rate and respiratory rate to determine animal’s state of health. A decrease or increase of the physiologic parameters from their normal ranges may quantify magnitude of a disease (Morgante et al., 2007).
The objective of this study was to evaluate the effect of rumenocentesis with aspirating long needle on some physiologic parameters of Yankasa Sheep. The specific aims were to determine accessibility of rumen liquor with aspirating long needle for rumen liquor pH evaluation and protozoa motility test in Yankasa Sheep; establishing a tool for acquiring this liquor for diagnosis of ruminal metabolic disorders or nutritional analysis.
MATERIALS AND METHODS
Study Area
The study was conducted in the Animal Science Research Farm of the University of Maiduguri, located in Maiduguri the capital of Borno State. Maiduguri is situated in the North-Eastern region of Nigeria at latitude 11.50N and longitude 30.050E the Sudan-Savannah (Udo, 1978). The city has a population of about 1,197,497 as of 2007 and holds a significant livestock population in the country Nigeria.
Study Animals
Ten clinically healthy Yanksa Sheep used for the study were provided by the Department of Animal Science and the experiment was approved by the Animal Ethic Committee of the Department of Veterinary Surgery and Radiology. The Yankasa Sheep used in this study were intensively managed during the whole research period and baseline values were used as control. Feed and water were provided ad libitum. Animals were allowed to acclimatize for a period of one month before commencement of the experiment. Blood samples (5mls) each were drawn from the jugular vein into an anti-coagulant, ethylene diaminetetracetate (EDTA) bottle and transported in an ice pack for haematology in Clinical Pathology Laboratory of the Department of Veterinary Pathology, University of Maiduguri.
Pre-Operative Preparation/ Anaesthetic Induction
Animals were allowed feed and water ad libitum. Each animal was physically restrained in a standing position, the dorsal surface of the caudal part of the rumen located slightly ventral to the paralumbar fossa on the left side of the sheep was shaved using a razor blade. The shaved area was aseptically prepared with 4% chlorhexidinegluconate (Savlon®,Veraadingdeur, Johnson and Johnson (pty) Ltd, London). Local anaesthetic 2% lidocaine HCL 4mg/kg (Lidocaine®,kwality pharmaceuticals(P) Ltd. Nag Kalan,Majitha Road, Amristar,INDIA) was infiltrated in a ring block method around the surgical site and allowed for 2 minutes to take effect while further scrubbing of surgical site was done using methylated spirit.
Rumenocentesis
The aspirating long needle of an automatic syringe (ALN) was attached with aspirating liquid ingress tube (ALIT) to its distal end, and a 10mL syringe attached to the end of the ALIT (Figure 1). Following aseptic preparation and a local infiltration of the left paralumbar fossa with antiseptic and lidocaine respectively, fifteen centimetres (15cm) of ALN was inserted into the dorsal rumen and a putrid gas was expelled indicating the rumen was penetrated. Five millilitres (5ml) of the rumen fluid was slowly aspirated and turned into a plastic container and immediately the pH of the rumen fluid was measured using a pH meter, read and recorded. After each procedure, the ALN, ALIT and container were flushed with normal saline solution (Juhel®,Fabrique par juhel Nig. Ltd/ Awka, Anambara, Nigeria) to avoid alteration in pH of the rumen fluid. Collected rumen fluid were emptied into a plain sample bottle and conveyed to the Parasitology Laboratory of the Department of Veterinary Microbiology and Parasitology, University of Maiduguri, for protozoa motility test. Blood samples were collected at days 2, 3, 4, 5, 6 and 7 post rumenocentesis, when each Sheep was restrained in a standing position and jugular vein aseptically swabbed. Five millilitres (5ml) of blood was drawn into EDTA sample bottle and the blood was dispensed gently into the bottle and conveyed in an ice pack to the laboratory for haematological analysis. The post-operative care was five days systemic antibiotics, penicillin G Procaine 200,000 iu- Dihydrostreptomycin Sulphate (Penstrep®Mova Pharmaceuticals corporation Caguas, puertrico, Animal health care product ANOPCO, Ltdcrockatt Rd, Hadleigh, United Kingdom) was infiltrated around the surgical site and given intramuscularly. Analgesia was provided with Diclofenac sodium injection (Diclofenac Sodium®, Embassy pharmaceutical and chemical Ltd. Lagos Nigeria) for 3 days.
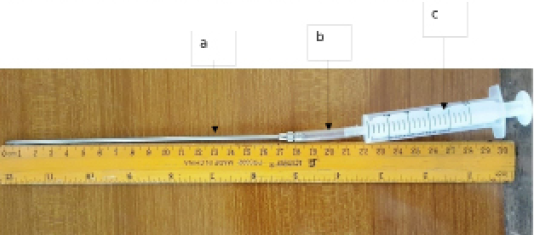
Figure 1: Aspirating long needle of an automatic syringe attached to ingress tube and syringe; a: aspirating long needle; b: ingress tube; c: syringe
Clinical parameters which include rectal temperature, heart rate and respiratory rate were taken with thermometer, stethoscope and visual observations respectively at sampling periods. The automatic thermometer was set to zero and the tail of the animal raised so that the thermometer was inserted gently into the rectum and tilted to an angle to touch the rectal wall. A sound was made displaying the rectal temperature on the thermometer screen, its readings were noted and recorded. Thermometer was disinfected after each animal’s body temperature was taken. The heart rate was taken by placing a stethoscope on the left side of the thoracic cavity between the sixth to tenth ribs. Respiratory rate was taken by carefully observing the rising and denting of the paralumber fossa.
Protozoa Motility
A drop of rumen fluid was put on a glass slide, covered with a cover slip and viewed with a microscope (Olympus) under a 10 x 10 magnification for protozoa motility.
Haematology
Haematological indices were evaluated using routine laboratory methods. Packed cell volume (PCV) were determined by the microhaematocrit method (Coles, 1986). Erythrocytes (red blood cells), Leucocytes counts (WBC) and Haemaglobin (Hb) were evaluated as described by (Brown, 1976).
Statistical Analysis
Generated data were analysed using graph pad prism version 5.03 (2005). Evaluating mean±SEM (m±SEM) in Column Statistics and One Way Repeated Measures ANOVA with Dunnett’s Post Test was employed.
RESULTS AND DISCUSSION
Aspirating long needle rumenocentesis was successful and convenient to Yankasa Sheep. The animals survived the procedure without any complications. The m±SEM of the rumen liquor pH was 6.3±0.10 (data not shown) which is slightly below normal range 6.4-6.8 (Bassert, 2010). Rumen liquor samples showed positive protozoa motility that was rated or scored 100% (data not shown). The haematology indices (Table 1) are that of baseline and post-rumenocentesis results of Yankasa Sheep used in the study. The baseline PCV was 28.9±0.38% within normal reported range for ovine species (Williams and Wilkins, 2000). The days 1 and 2 PCV values were 32.7±1.24%, and 32.7±1.04% respectively, significantly different (p<0.05) compared to the baseline. Although there was a slight increase in the values of the PCV at subsequent days 3, 4, 5, 6 and 7 post-rumenocentesis compared to the baseline, the values at different days showed no significant difference compared to the baseline (p>0.05). HB values were significantly different at all days post rumenocentesis except at day 5 (10±0.21 g/dl) compared to baseline (10±0.25 g/dl). The RBC, monocytes and basophil values at different days post rumenocentesis were not significantly different (p>0.05) with that of the baseline (12.20±0.49 x106μl, 0.01±0.01 x106μl, 0.00±0.00 x106μl. The days 1 and 4 WBC values 11.20±0.43x103μl and 10.9±0.37x103μl respectively post rumenocentesis were significantly different (p<0.05) from the baseline (9.61±0.59x103μl). The neutrophil
Table 1: m±SEM haematological profile of Yankasa Sheep post rumenocentesi
|
Evaluated periods (days) |
||||||||
|
Variable |
Baseline |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
Day 7 |
|
PCV (%) |
28.9±0.38a |
32.7±1.24b |
32.7±1.04b |
29.4±0.84a |
30.6±1.08a |
26.6±1.08a |
28.8±0.93a |
28.7±0.99a |
|
HB (g/dl) |
10±0.25c |
12.90±0.34d |
12.25±0.38d |
11.42±0.30d |
11.73±0.33d |
10±0.21c |
11.78±0.29d |
11.49±0.36d |
|
RBC (x106µl) |
12.20±0.49e |
13.00±0.39e |
13.00±0.49e |
11.50±0.32e |
13.40±0.39e |
11.30±0.41e |
12.20±0.28e |
11.70±0.28e |
|
WBC (x103µl) |
9.61±0.59f |
11.20±0.43g |
10.2±0.43f |
10.6±0.36f |
10.9±0.37g |
10.6±0.20f |
9.74±0.18f |
10.2±0.25f |
|
NEUT (x103µl) |
4.67±0.31h |
3.14±0.12i |
3.23±0.14h |
3.47±0.09h |
3.86±0.07h |
4.26±0.71h |
3.37±0.11h |
3.18±0.09i |
|
LYMP (x103µl) |
4.18±0.31j |
6.40±0.27j |
5.56±0.32j |
6.13±0.36j |
9.18±0.45k |
7.18±1.42j |
5.73±0.17j |
5.05±0.57j |
|
MONO (x103µl) |
0.01±0.01l |
0.38±0.03l |
0.35±0.04l |
0.36±0.04l |
0.41±0.04l |
0.39±0.03l |
0.43±0.03l |
0.97±0.61l |
|
EOSI (x103µl) |
0.18±0.09m |
1.10±0.12n |
0.85±0.24n |
0.76±0.09n |
0.75±0.06n |
0.83±0.05n |
0.65±0.08n |
0.77±0.03n |
|
BASO (x103µl) |
0.00±0.00o |
0.02±0.01o |
0.00±0.00o |
0.00±0.00o |
0.00±0.00o |
0.00±0.00o |
0.01±0.01o |
0.01±0.01o |
Values with different superscripts within rows differ significantly (p<0.05) to its respective baseline; PCV: Packed cell volume; HB: Haemoglobin; RBC: Red blood cell; WBC: White blood cell; NEUT: Neutrophils; LYMP: Lymphocytes; MONO: Monocytes; EOSI: Eosinophils; BASO: Basophils
Table 2: m±SEM clinical/physiological parameters of Yankasa Sheep post rumenocentesis
|
Evaluated periods (days) |
||||||||
|
Variables |
Baseline |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
Day 7 |
|
Heart rate (Beats/ Min) |
79.70±0.86a |
78.00±0.77a |
75.8±1.98a |
73.80±0.93a |
77.3±0.97a |
78.8±1.17a |
78.6±1.39a |
76.2±1.54a |
|
Respiration rate (Breaths/Minute) |
29.20±1.57b |
28.10±0.57b |
28.00±1.69b |
29.40±1.12b |
29.20±1.58b |
28.00±1.69b |
27.7±1.57b |
28.10±1.29b |
|
Rectal temperature(0C) |
38.40±0.11c |
38.30±0.18c |
38.30±0.17c |
38.60±0.09c |
38.40±0.08c |
38.40±0.77c |
38.40±0.10c |
38.3±0.088c |
Values with same superscripts within rows do not differ significantly (p>0.05) to the baseline values
values for days 1 and 7 were 3.14±0.12 x103μl, and 3.18±0.09 x103μl and were significantly different from that of the baseline (4.67±0.31 x103μl). The lymphocyte value at day 4 (9.18±0.45) was significantly different from the baseline (4.18±0.31 x103μl). The values of the eosinophil at all days are significantly different from the baseline (0.18±0.09 x103μl). The baseline values for the heart rate, respiratory rate and temperature were 76.70±0.86 beats/min, 25.20±0.57 breath/min, 38.40±0.110C and showed no significant difference with the values post rumenocentesis (p>0.05) as indicated in Table 2.
The Yankasa Sheep used in the experiment, recovered from the procedure without any serious local skin tissue reaction (Tajik et al., 2011). The puncture site was the dorsal sac of the rumen through the left paralumbar fossa between the tuba coxae and the 13th rib, thereby preventing rumen content leakage as opposed to the ventral technique (Enemark et al., 2004). Peritonitis and haematoma were reported in some studies (Strabel et al., 2007) this study revealed no such problems and could not be unconnected to the strict post-operative antibiosis, analgesia and the anatomic puncture site of the skin and the rumen. Although the M±SEM of the rumen pH in this study was slightly lower 6.3±0.10 compared to the reference value 6.4-6.8 (Bassert, 2010), protozoa motility test was positive. This indicates that at pH 6.3, rumen flora (protozoa) are not affected by the slight drop in rumen liquor pH. Packed Cell Volume increased significantly at days one and two when compared to the baseline value, it did not differ from reference range of the ovine species (27-45%) (Williams and Wilkins, 2000). Pain following rumenocentesis can cause anorexia and as such animals will not eat and drink as required (Abdallah et al., 2010). This can lead to decrease in plasma volume and subsequent increase in the pack cell volume as seen in conditions such as dehydration (Abdallah et al., 2010). The decrease in plasma volume observed in this study is responsible for the increase in haemoglobin concentration at the different sampling periods although the values are within reference range (15g/dl) reported by (Egbe et al., 2000). The red blood cell, monocyte and basophil were not affected (p>0.05) following aspirating long needle rumenocentesis. The significant difference (p<0.05) in white blood cell at days 1 and 4 to the baseline is due to neutrophilia at day 1 and lymphocytosis at day 4. The plausible explanation is the mobilisation of cells due to fright, stress and inflammation. Stress induce the nerve cells to release noradrenalin, and further signal the bone marrow as neurotransmitter, to produce haematopoietic stem cells which can become any type of cell, under stress condition (Morag, 2002). The haematopoietic stem cells are converted to white blood cell resulting to increase number of white blood cell. Inflammation is the animal protective response aimed at eliminating the cause of cell injury and initiating the process of tissue repair during these process white blood cells is mobilised to the site of injury or tissue damage to prevent infection as seen in this procedure. During fright epinephrine is released and this cause release of cells usually neutrophils resulting to a rise (Morag, 2002). The significant difference at day 7 could be attributed to the onset of inflammation at the puncture site following stoppage of postoperative analgesics and antibiotics at days 3 and 5 respectively (Morgante et al., 2007). The significant difference in eosinophil compared to the baseline is due to tissue injury leading to degranulation of mast cells and histamine release. Histamines are chemotactic to eosinophil, therefore eosinophil are mobilised from the bone marrow to the site of injury (Morag, 2002). Aspirating long needle rumenocentesis has no effect on the clinical parameters of Yankasa sheep because significant differences were not recorded (p>0.05) in this study, therefore aspirating long needle rumenocentesis is safe and reliable without the problems of needle luminal blockages as observed with 14G needles.
CONCLUSION
Haematological parameters were affected following aspirating long needle rumenocentesis among Yankasa Sheep but did not pose a threat to the animal. Therefore, aspirating long needle rumenocentesis was found to be a safe, fast, reliable and convenient technique of obtaining rumen liquor. Further studies should be conducted assaying biomarkers of inflammation and stress such as acute phase proteins and cortisol. Assaying markers of muscle damage such as creatine kinase, aspartate aminotransferase, aldolase and myoglobin may be necessary to have comprehensive reports on the invasive extent or muscle damage that may be associated with aspirating long needle rumenocentesis.
ACKNOWLEDGEMENT
The authors are thankful to the Department of Animal Science, University of Maiduguri, for providing the research animals.
CONFLICT OF INTEREST
There was no conflict of interest among the authors of this research article.
AUTHORS CONTRIBUTIoN
All authors participated in writing and/or editing the manuscript of this research article while specific roles were: Abubakar Mshelia Saidu designed the experiment, performed surgeries, collected samples and provided post-operative care, Jummai Ilebaye Abbah collected and assays samples provided post-operative care, Usman Mohammed Kolo designed the experiment and collected samples, Dauda Laku and Musa Kalim Adam performed surgeries.
REFERENCES






